1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

How do Van der Waals constants a and b depend on temperature, pressure and volume? - Quora
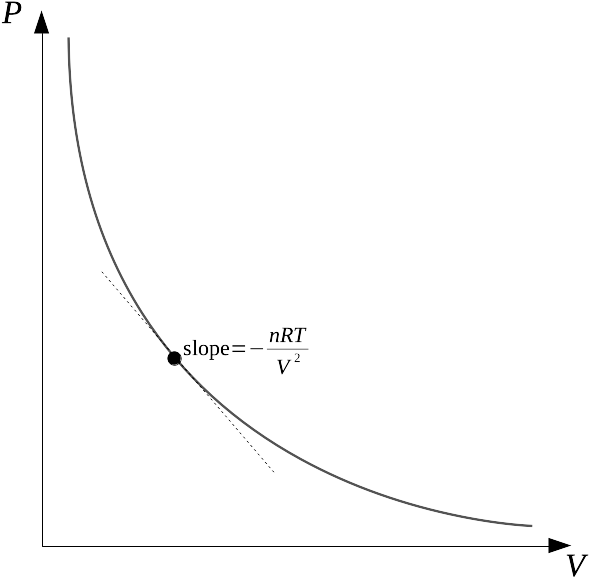
1.8: The ideal gas law, functions and derivatives - Chemistry LibreTexts

Van Der Waals Equation of State - an overview

Boyle Temperature for Van der Waals, Berthelot and Dieterici, Unit 2, BPC Class

Van der Waals Equation Practice Problems
Solved] The van der Waals equation of state was designed (by Dutch
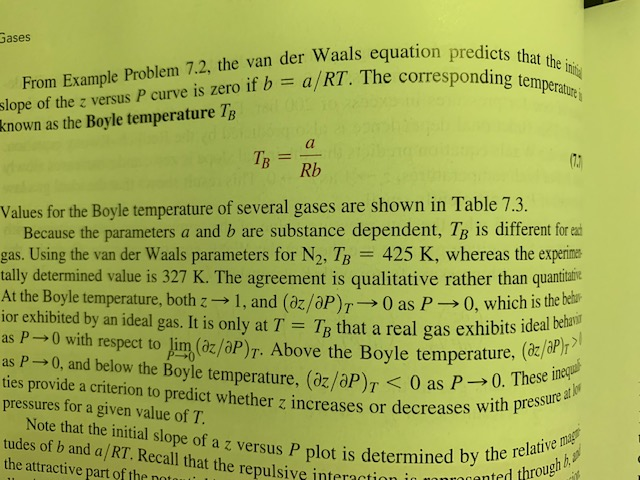
Solved the Boyle temperature TB is an important parameter

Solved The van der Waals equation of state written in terms

The Boyle temperature of a van der Waal gas is `-246^(@)C`. Its critical temperature on absolute

Van der Waals Equation Practice Problems
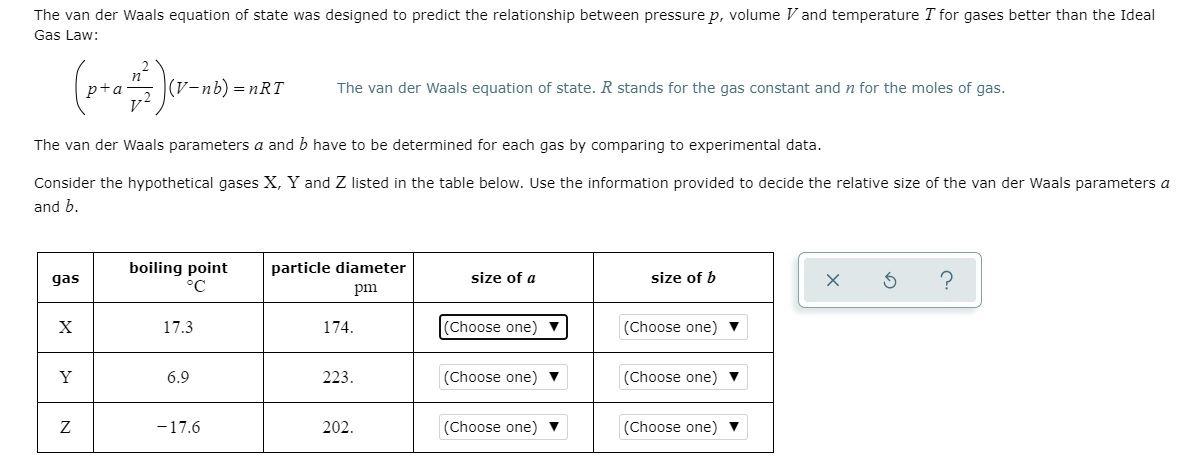
Solved The van der Waals equation of state was designed to