Physical Chemistry The Compression Factor (Z) [w/1 example]

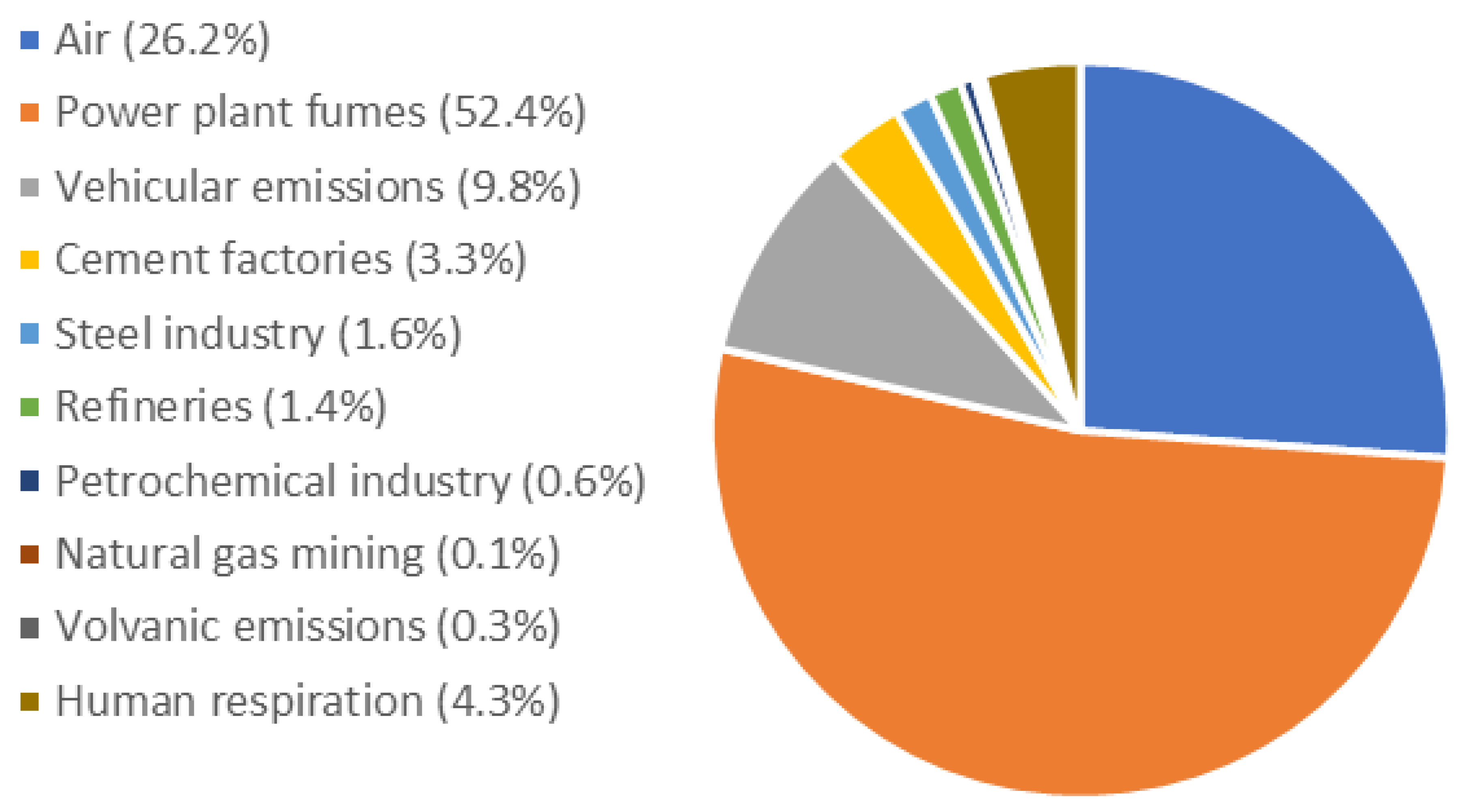
Atmosphere, Free Full-Text

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Physical Chemistry The Compression Factor (Z) [w/1 example]

Compressibility Factor Calculator

Compressibility Factor Z Important Concepts and Tips for JEE Main

Physical Chemistry The Compression Factor (Z) [w/1 example]

Real gas 1.molecules not always in motion (condense phase can be formed) 2.molecular size is non-negligible (there is molecular repulsion) 3.Molecules. - ppt download

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
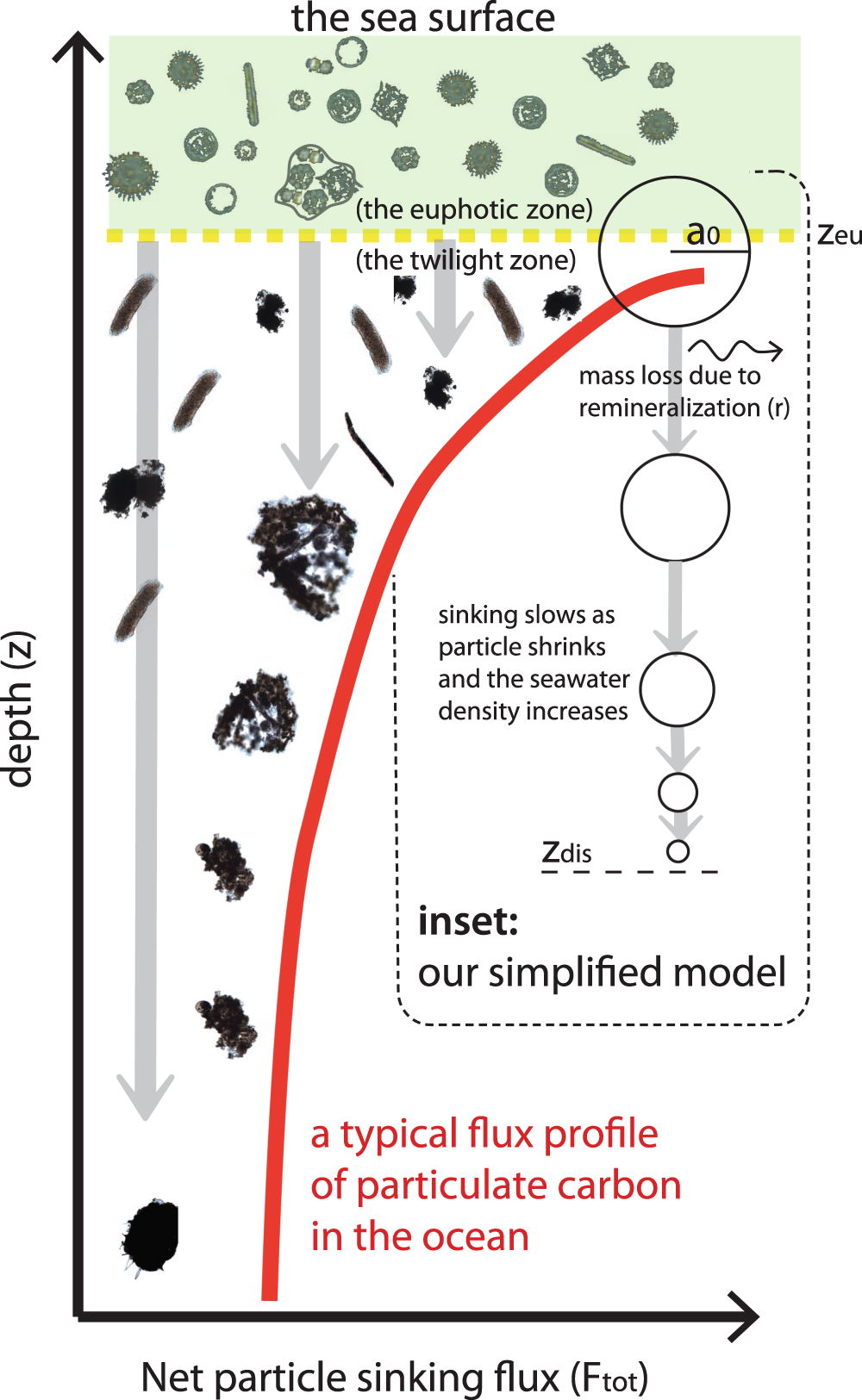
Sinking flux of particulate organic matter in the oceans: Sensitivity to particle characteristics

Physical & chemical properties finder

Compressibility factor - Wikipedia