At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

At low pressure, the van der Waals equation is reduced to

Van der Waals Equation, Definition & Examples - Lesson
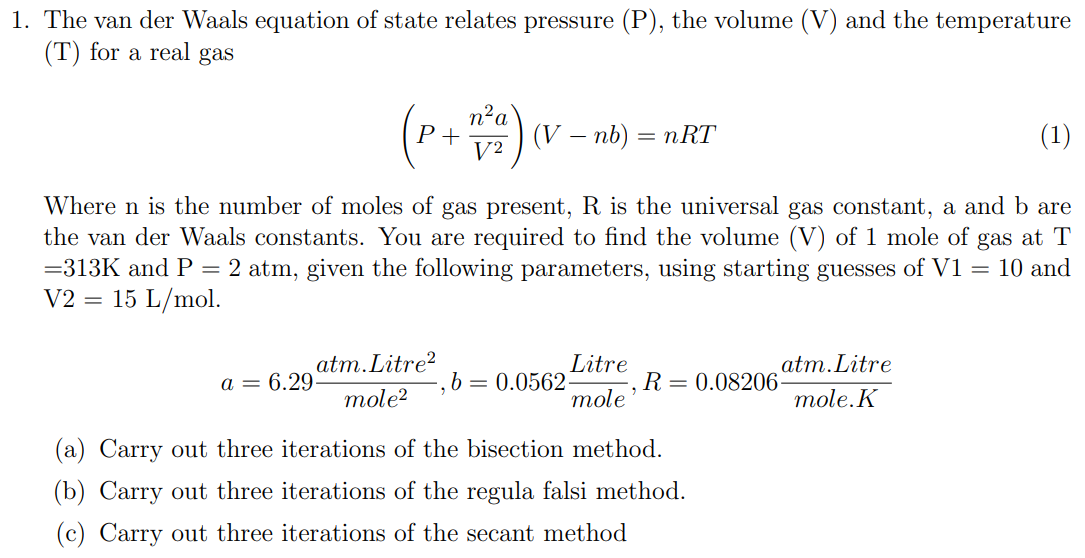
Solved The van der Waals equation of state relates pressure

Non-Ideal Gas Behavior

The van der Waals equation of the state is given as where, P
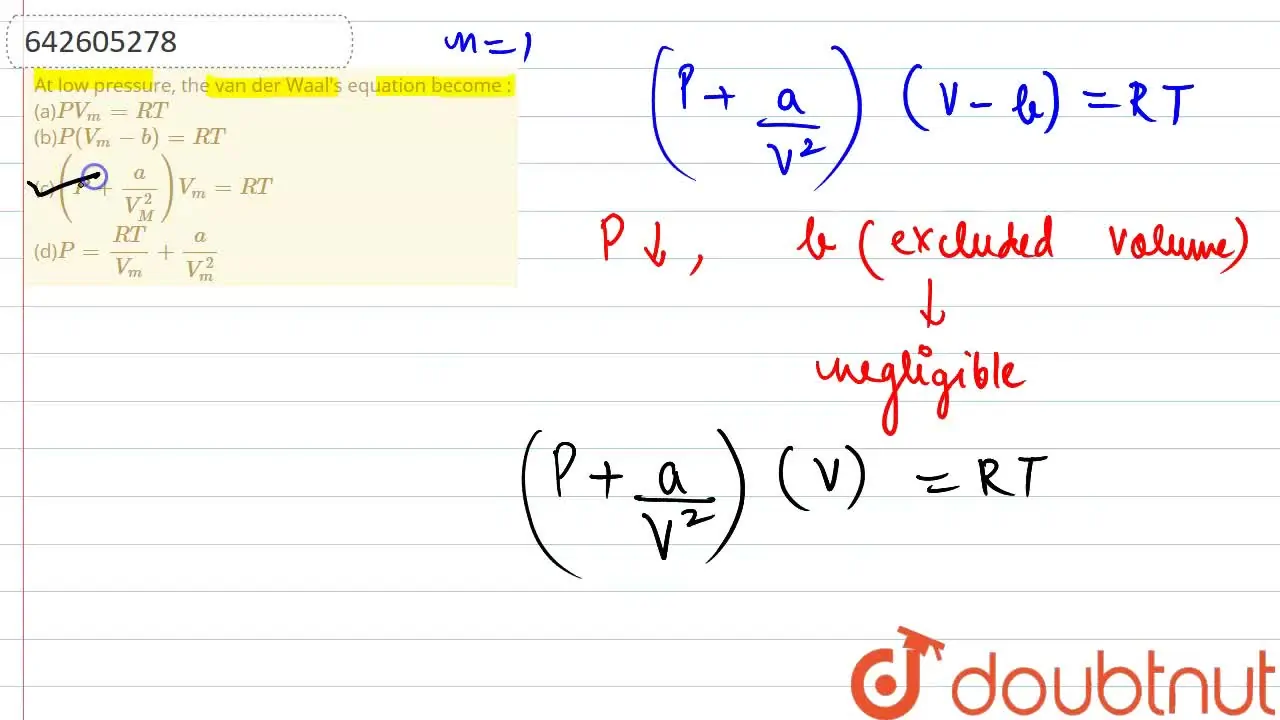
At low pressure, the van der Waal's equation become : (a)PV(m)=RT (b
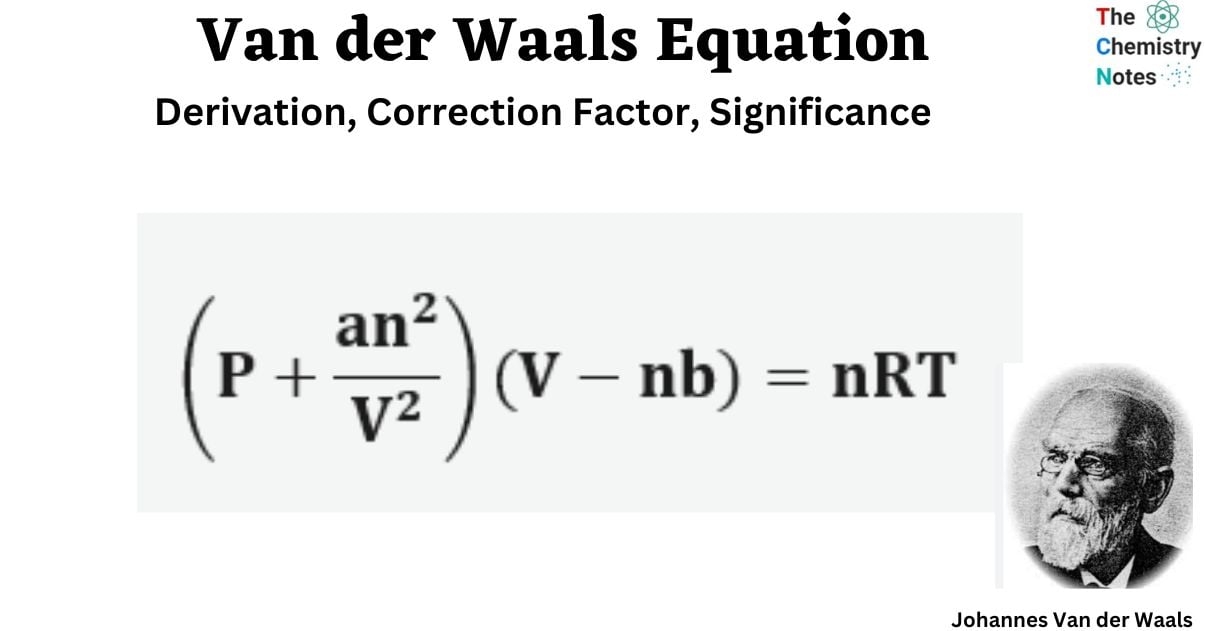
Van der Waals Equation: Derivation, Correction Factor, Significance

6.3: Van der Waals and Other Gases - Physics LibreTexts

At high temperature and low pressure, the van der Waals' equation
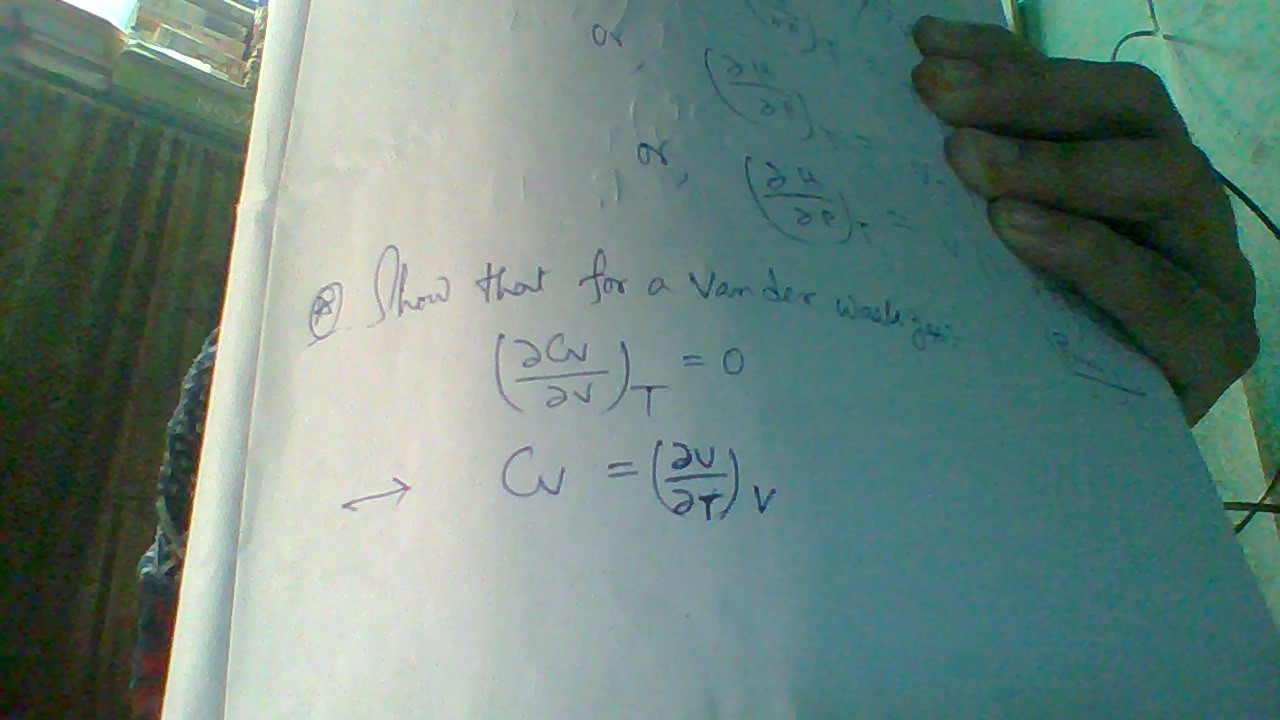
Show that for a van der Waals gas, ((delC_V)/(delV))_T = 0, where
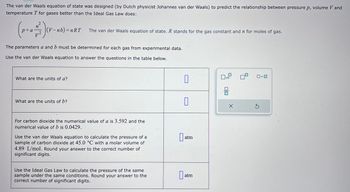
Answered: The van der Waals equation of state was…
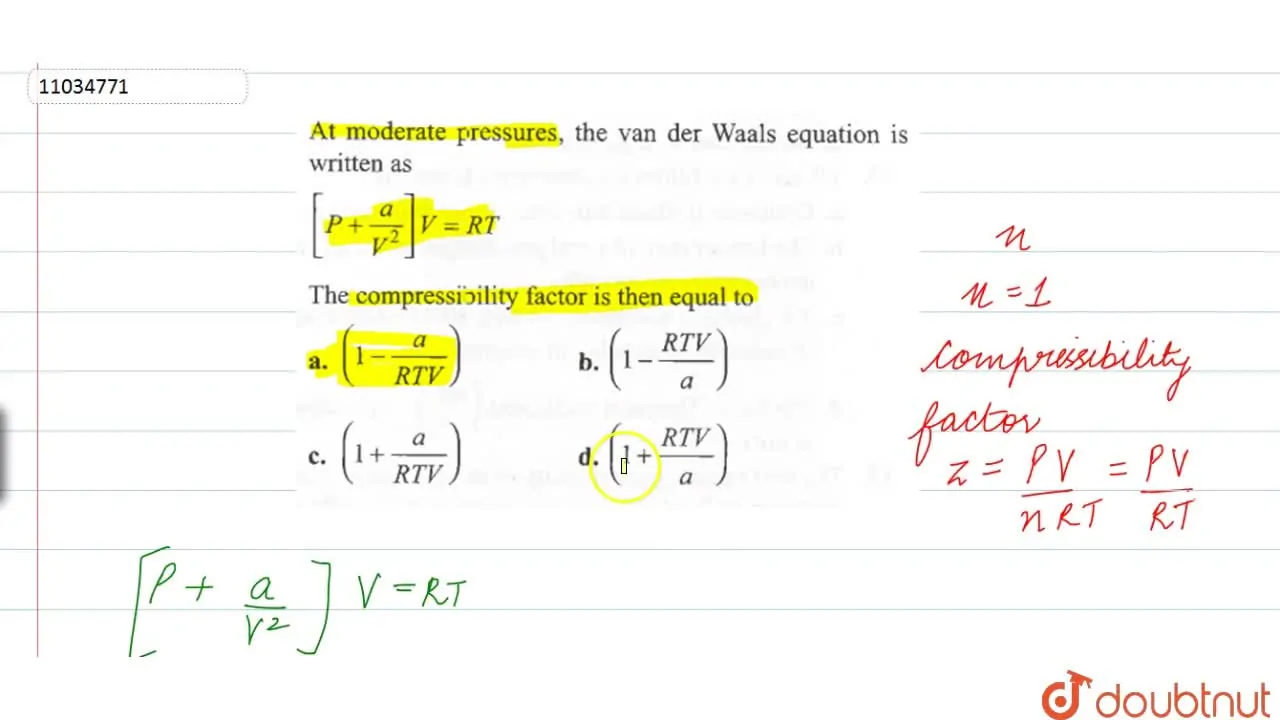
At low pressures, the van der Waals equation is written as [P+(a)/(V^(

Van Der Waals Equation - an overview