FDA Cleared vs Approved vs Granted for Medical Devices
Ever wonder what FDA cleared vs approved vs granted actually mean? Learn the subtle yet important differences between these regulatory terms.
USA regulatory process for medical devices

Understanding the Difference: FDA Cleared vs. Approved

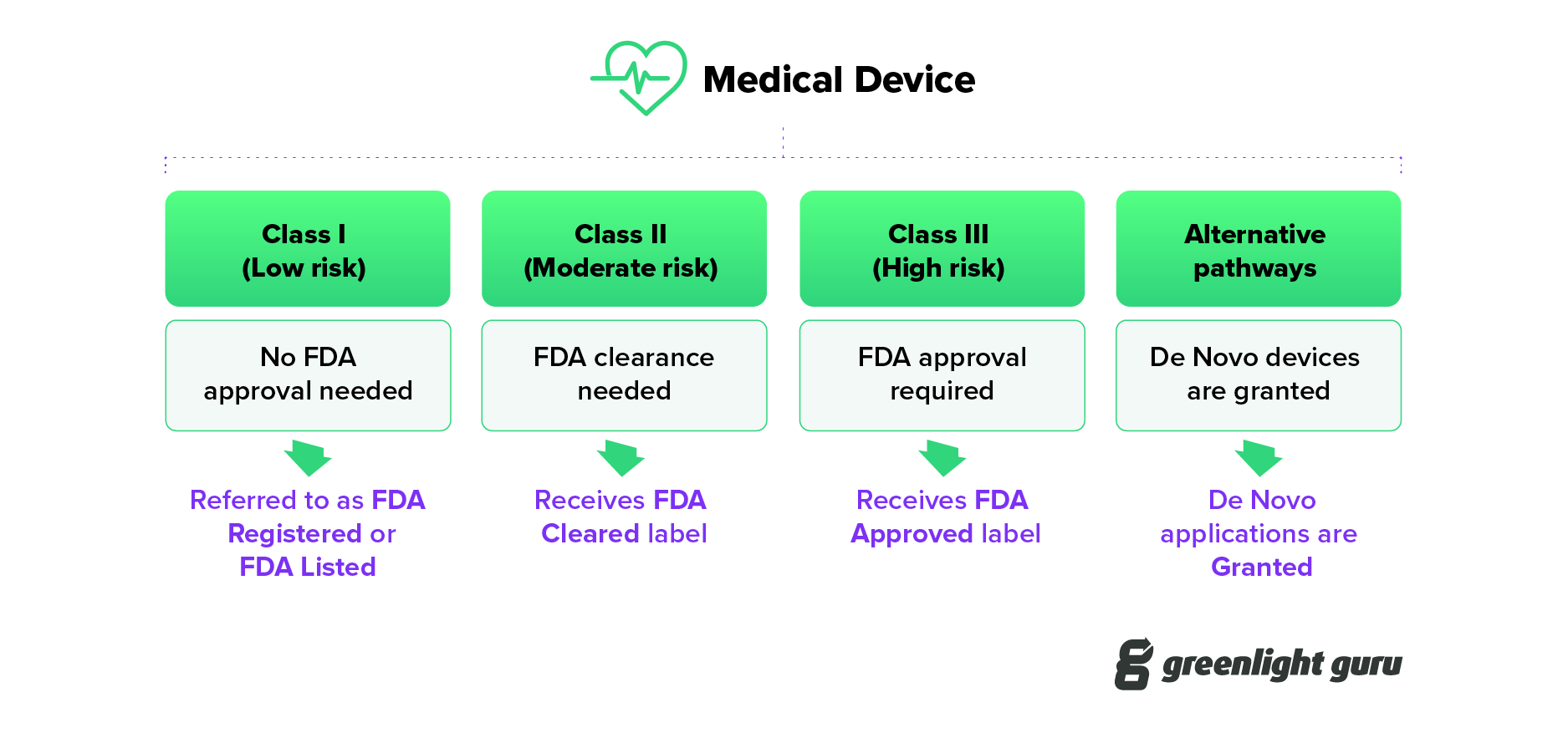
FDA listed, cleared, approved, granted - what IS the difference?

What is a Complete Response Letter?

FDA Device Regulation: 510(k), PMA · Academic Entrepreneurship for Medical and Health Sciences

Medical Device Development 101: Pathways to Clearance or Approval in the U.S. - Virginia Contract Research Organization Co., Ltd.

MillennialEYE Understanding On-Label, Off-Label, and Unapproved Products
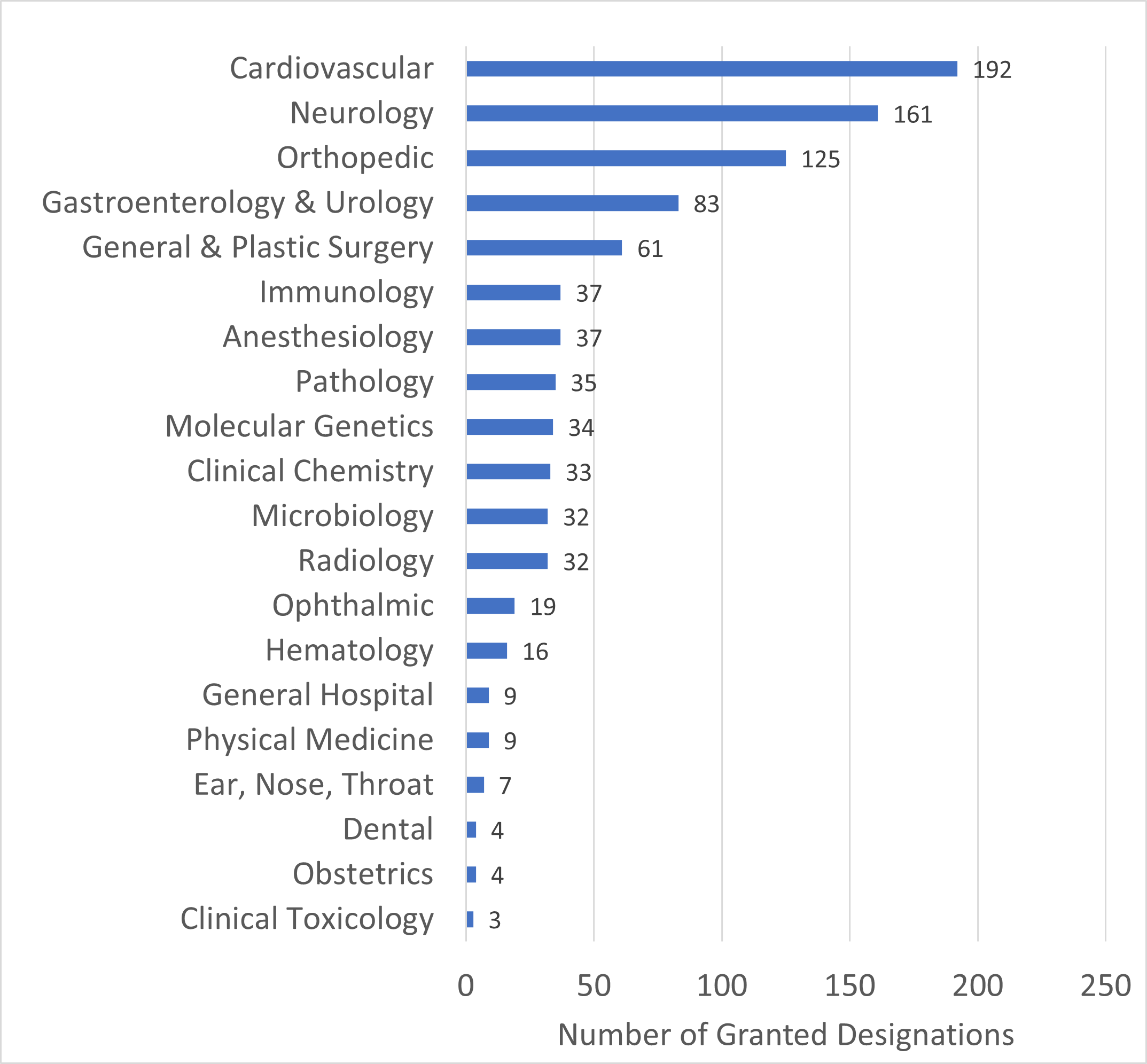
Breakthrough Devices Program

The Current State Of Almost 700 FDA-Approved, AI-Based Medical Devices

Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices - ScienceDirect

Understanding FDA Registered vs Cleared vs Approved vs Granted for Medical Devices
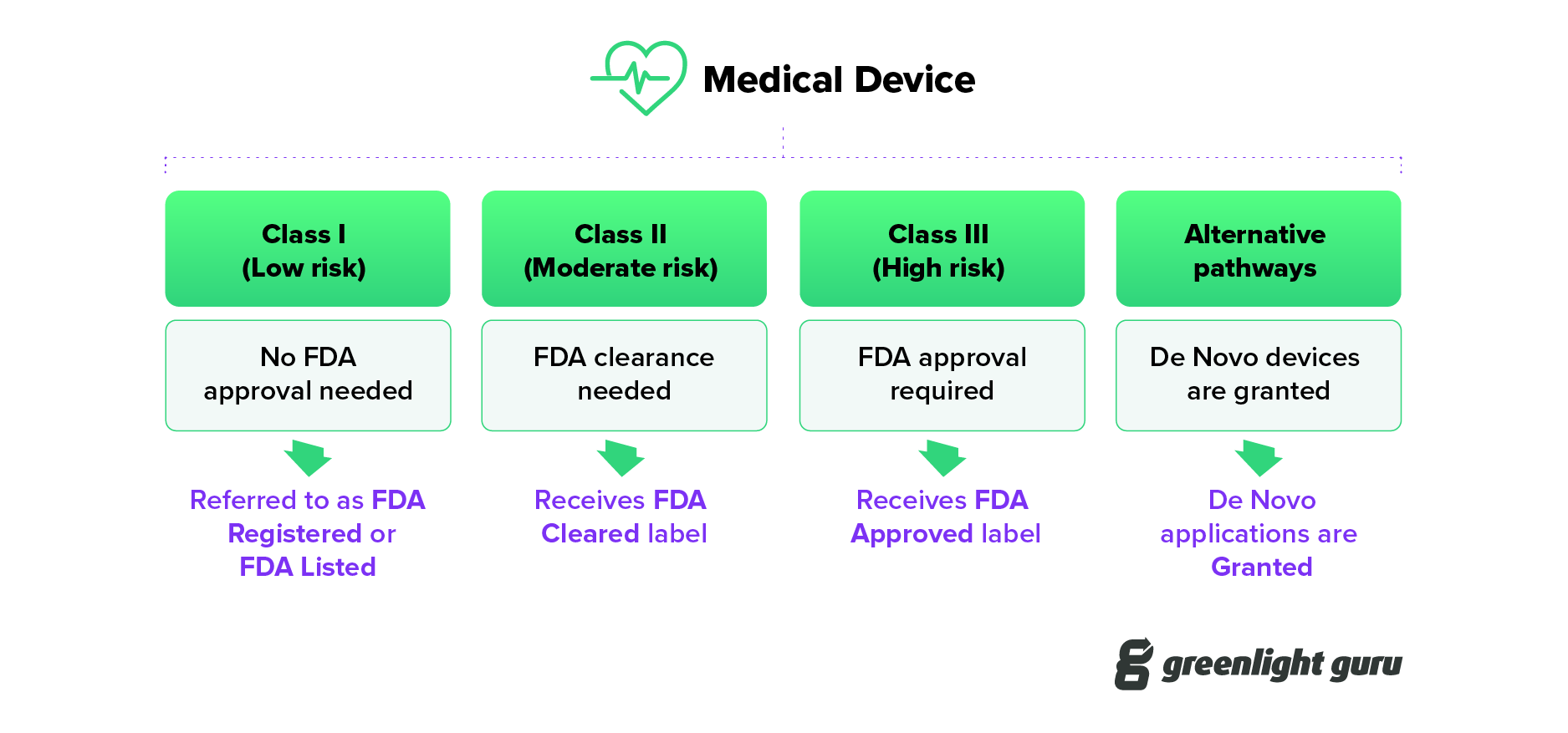
FDA Cleared vs Approved vs Granted for Medical Devices

Beyond the 510(k): The regulation of novel moderate-risk medical devices, intellectual property considerations, and innovation incentives in the FDA's De Novo pathway