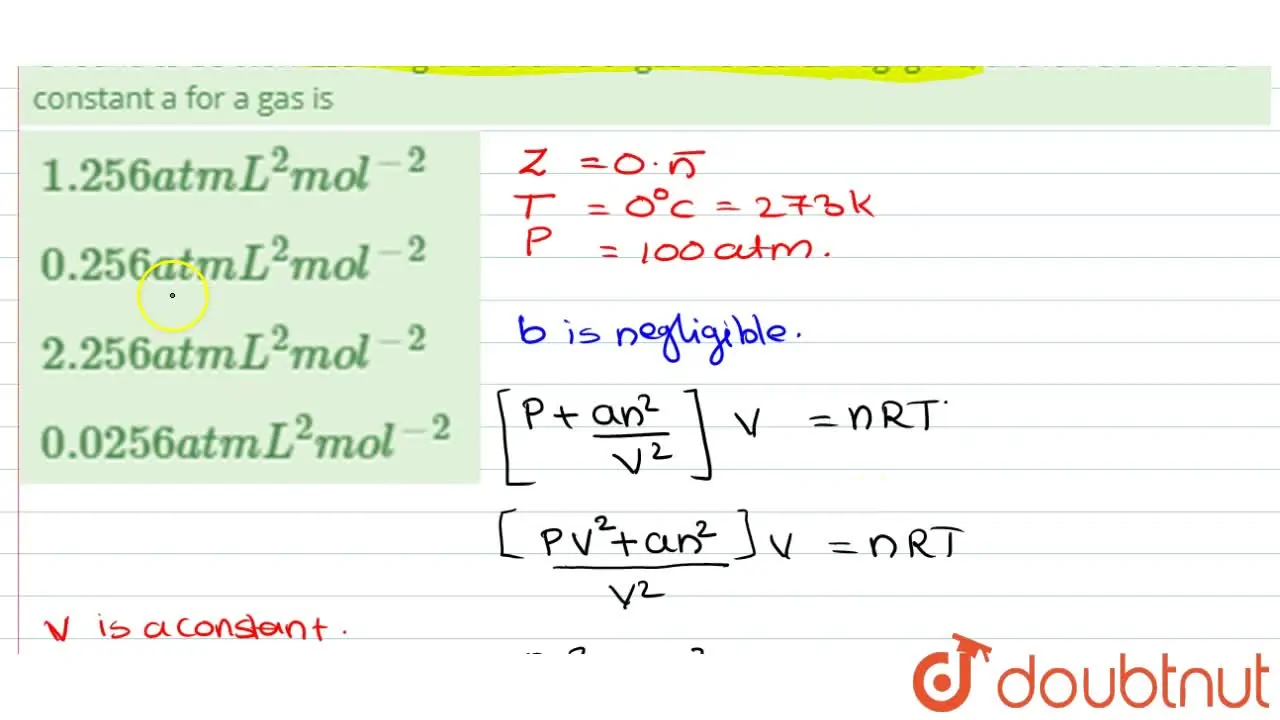
the compression factor one mole of a vander waals gas 0 C and 100

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor for one mole of a vander waals gas at 0 c and
Click here👆to get an answer to your question ✍️ The compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0-5
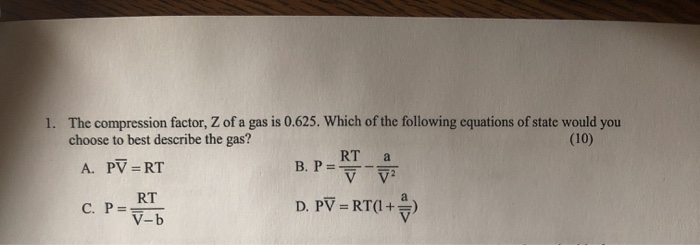
Solved 1. The compression factor, Z of a gas is 0.625. Which
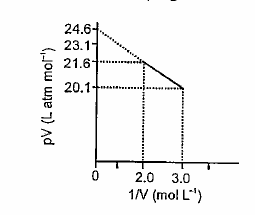
Bengali] For one mole of a van der Waals' gas when b = 0and T =300 K
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0ºC and 100 atm pressure is - Sarthaks eConnect

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

The compression factor (compressibility factor) for one mole of a Van der..

18. The compressibility factor one mole of a vanderwaal's gas 0°C and 100 atm pressure is found to be 0.5. Assume that the volume of gas molecule is negligible calculate the vanderwaals

the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5
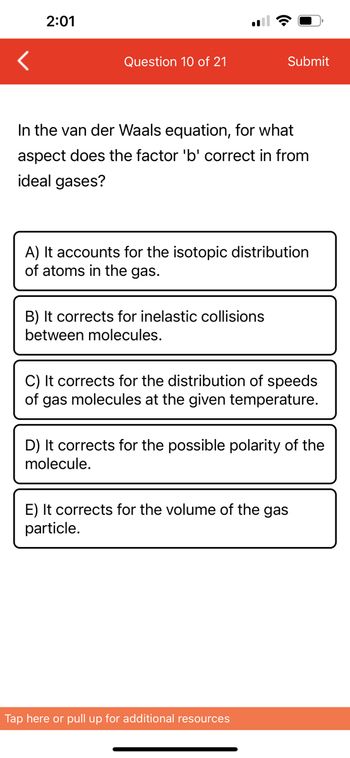
Answered: < 2:01 Question 10 of 21 il ☎ Sub In…

Solved Question 1) For water at 293 K and 1 atm, the
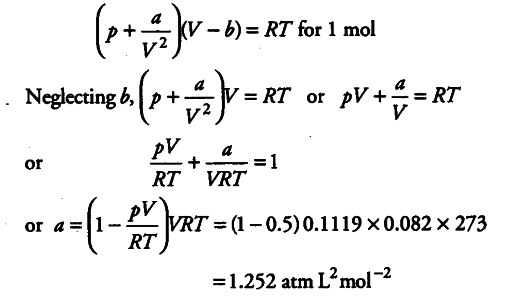
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum

Bengali] The compressibility factor (Z) of one mole of a van der Waal

Solved (a) The van der Waals model of liquid-gas systems
Malayalam] The compressibility factor for definite amount of van der

2 mol of ammonia occupied a volume of 5 L at 27∘ C. Calculate the pressure if the gas obeyed van der Waals equation. a = .4.17 atm L 2 mol 2