physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m
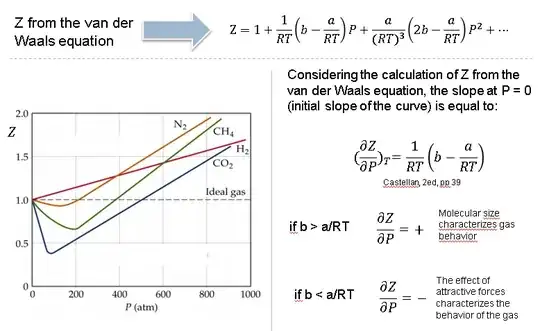
Why do some gases have lower value of Z for a particular pressure

A global equation-of-state model from mathematical interpolation
Virtual Particles: What are they? – Of Particular Significance
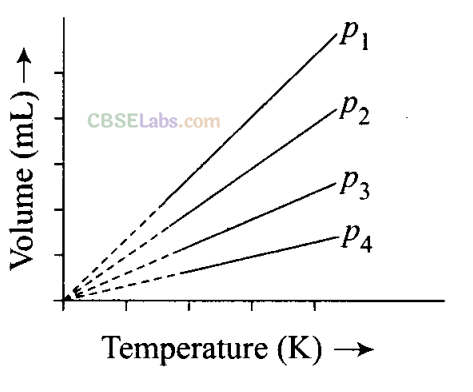
NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter
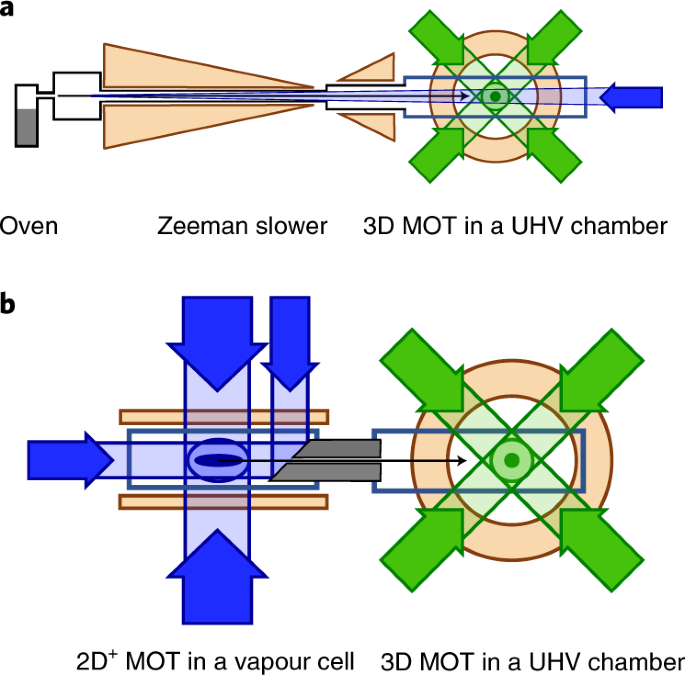
Laser cooling for quantum gases
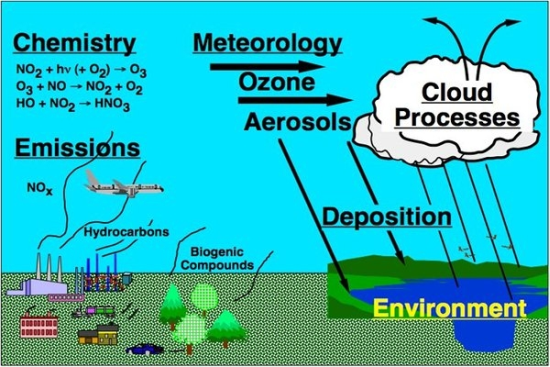
Atmosphere, Free Full-Text
Why would it be impossible for the pressure of the gas to be

Micromachines, Free Full-Text

History of climate change science - Wikipedia

Chemistry - Unit 3 - Joseph Flashcards
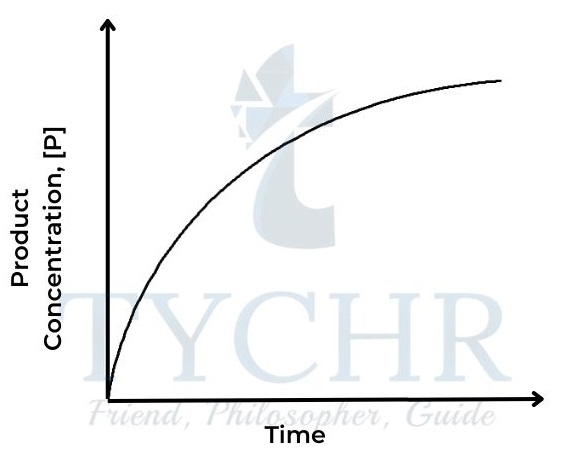
IB Chemistry, Chemical Kinetics Notes
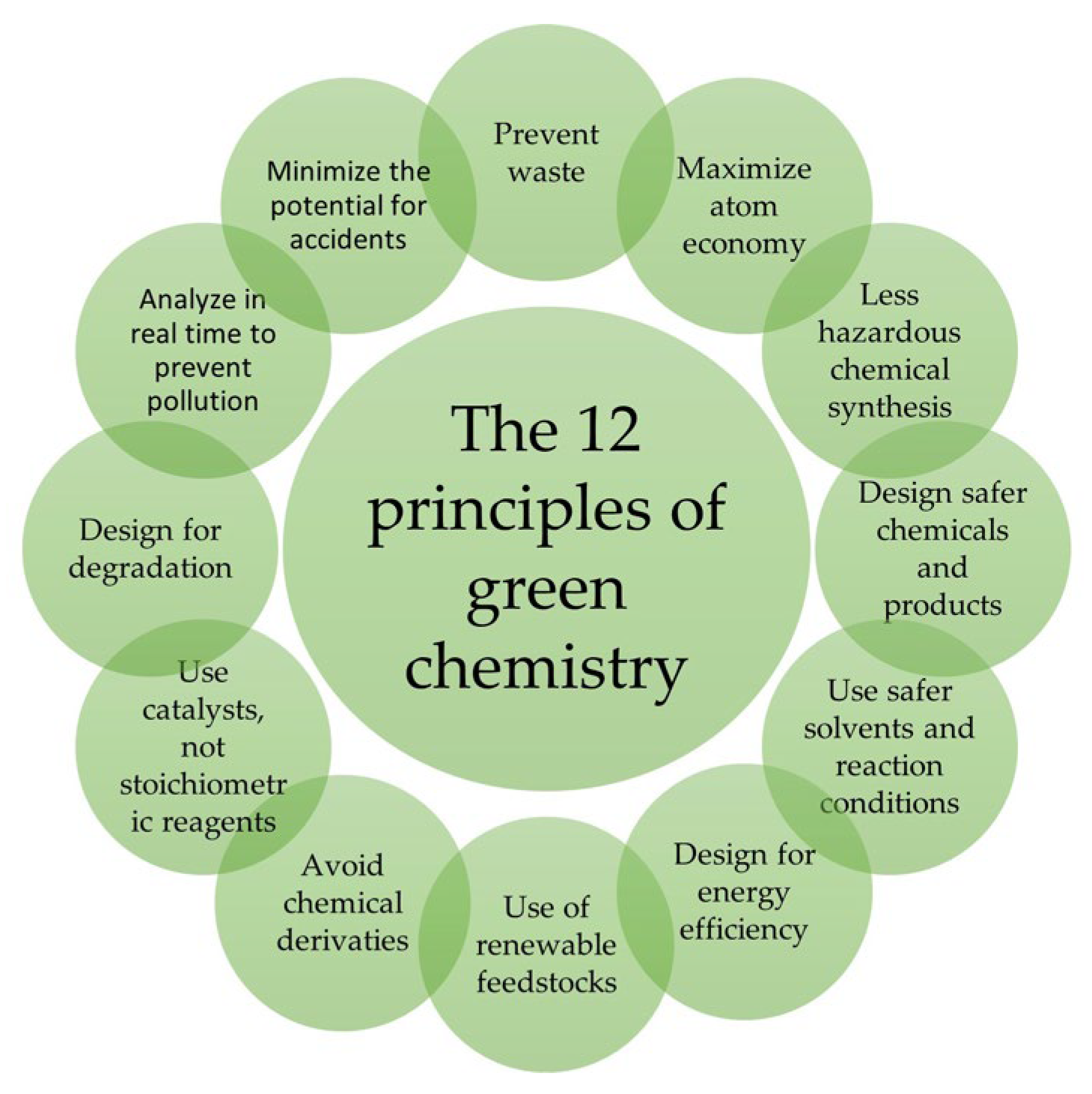
Molecules, Free Full-Text

The Conversion of Carbon Monoxide and Carbon Dioxide by