The vapour pressure of a solution having 2.0 g of solute X (gram
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8
What is the vapour pressure of a saturated solution of Ba(OH) 2 in an ATM? The vapor pressure of water is 23.8mmHg at 25 degrees. - Quora


A solution containing 8.6 g L^-1 of urea is isotonic with a 5 %(w / v) solution of unknown sol
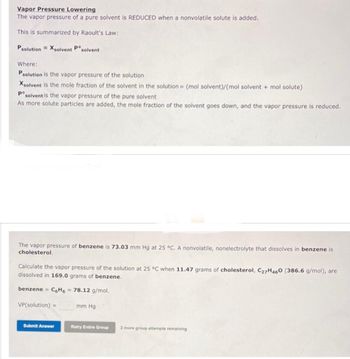
Answered: Vapor Pressure Lowering The vapor…

Volatile & Nonvolatile Solute Properties
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
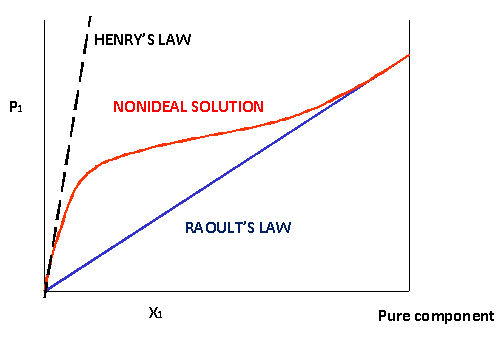
Raoult's Law - Chemistry LibreTexts

At 48°C the vapour pressure of pure CS2 is 850 torr. A solution of 2.0 g of sulphur in 100 g of CS2 has a vapour pressure 844.9 torr. Determine the atomicity

Ulal Mass) having 2.0 g of a mol-') in 100 g is 848.9 torr. The 22. The vapour pressure of a solution havine solute X (gram atomic mass = 32 g mol-1
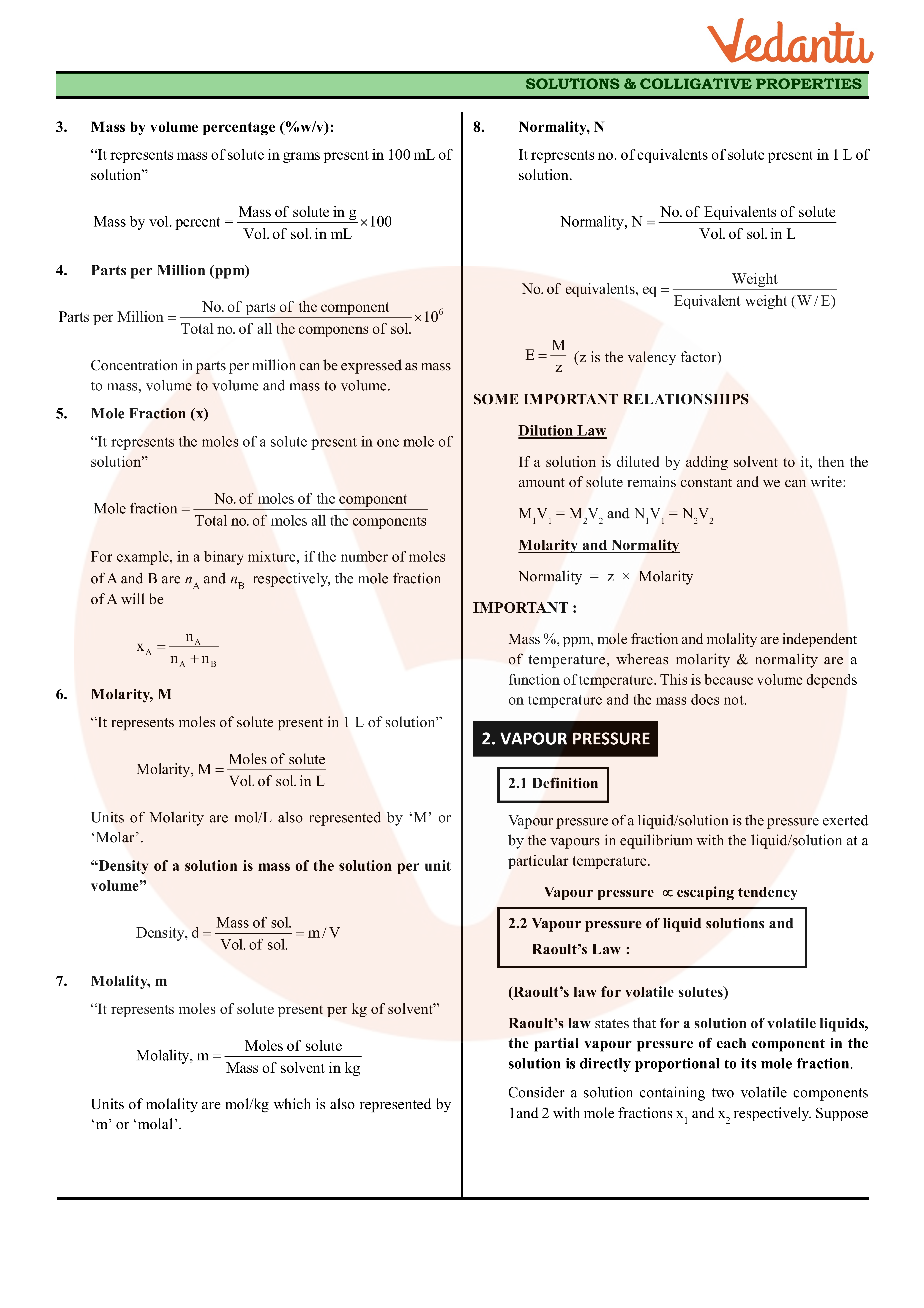
Solutions Class 12 Notes CBSE Chemistry Chapter 2 [PDF]