physical chemistry - Is the compressibility factor smaller or

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Real Gas Behavior The Compression Factor (Z) [Example #2]
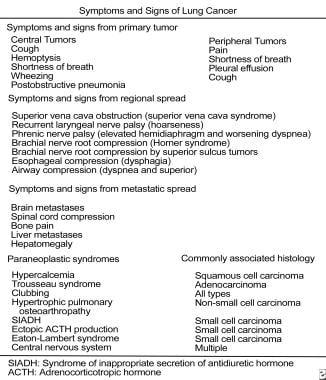
Non-Small Cell Lung Cancer (NSCLC) Clinical Presentation: History, Physical Examination

Gas compressibility factor Z: Ideal gas vs Real gas

States of Matter Class 11 Notes CBSE Chemistry Chapter 5 [PDF]

Compressibility Factor Charts - Wolfram Demonstrations Project

Compressibility CK-12 Foundation

Compressibility - an overview
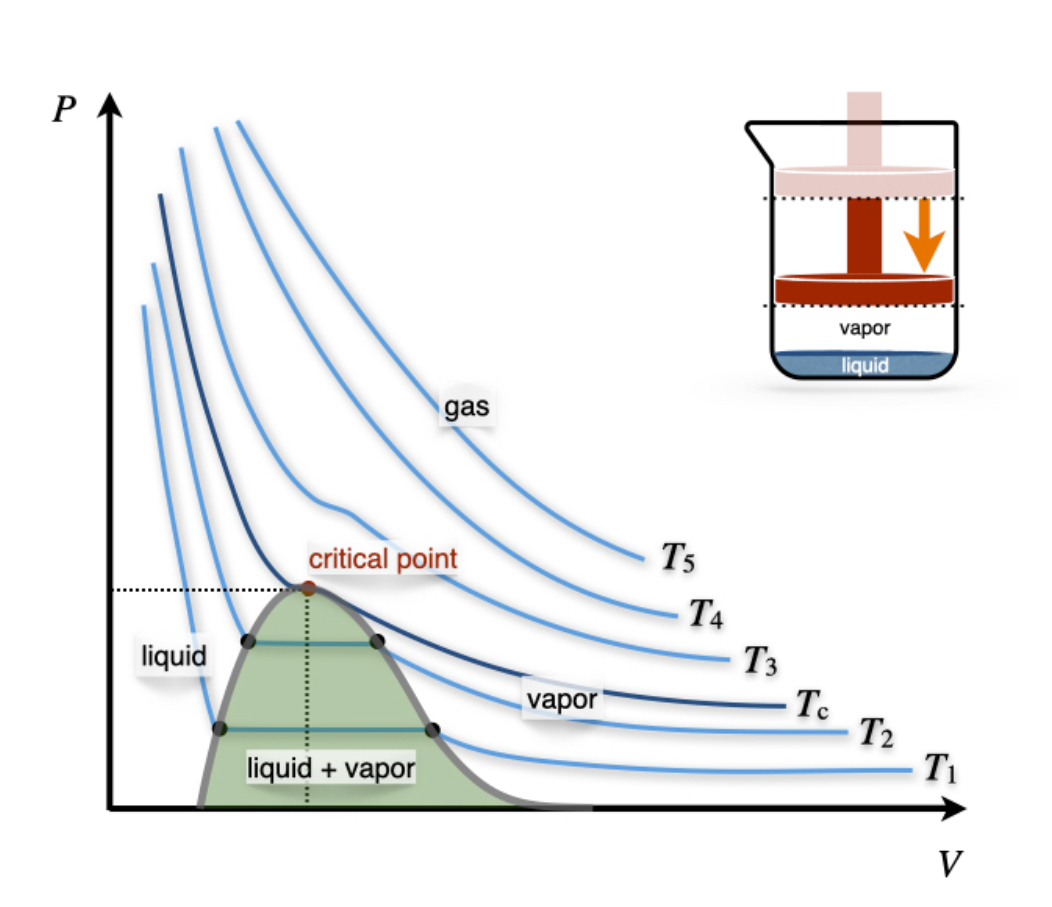
11.3: Critical Phenomena - Chemistry LibreTexts

Compressibility factor - Wikipedia

Reading Compressibility Factor Charts
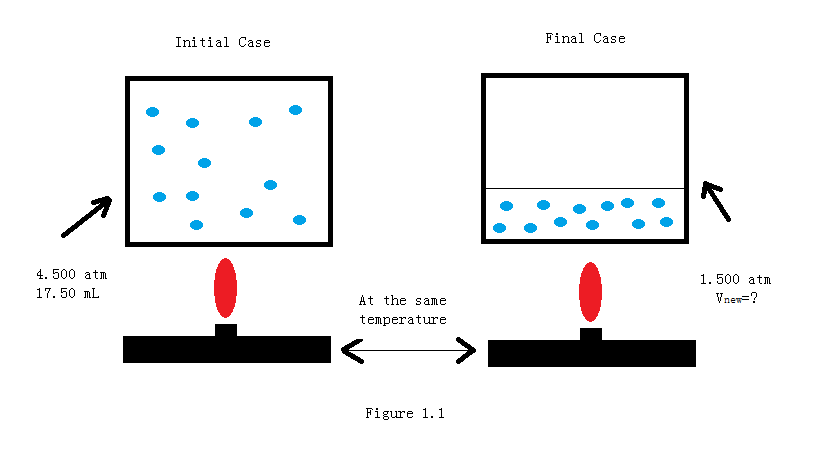
Gas Laws - Overview - Chemistry LibreTexts