First-in-human study of the safety, pharmacokinetics, and


Phase I, first-in-human study of XTR004, a novel 18F-labeled

Design of the first-in-human dose study with LY2457546. To

PDF) First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors

Denifanstat - Wikipedia

First in Human Subjects Enrolled in Aqualung Therapeutics Phase 1A
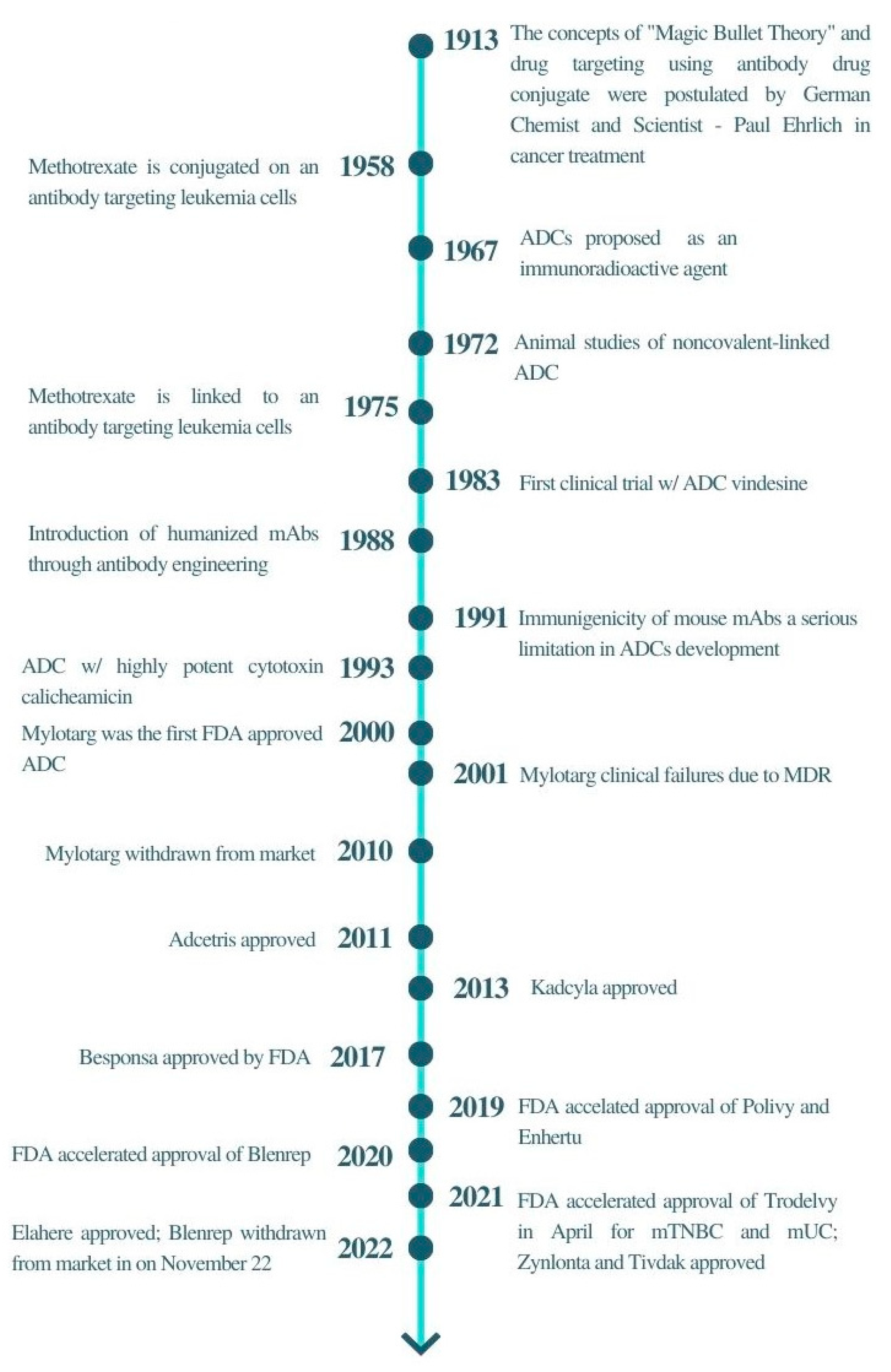
DDC, Free Full-Text

Biomedicines, Free Full-Text
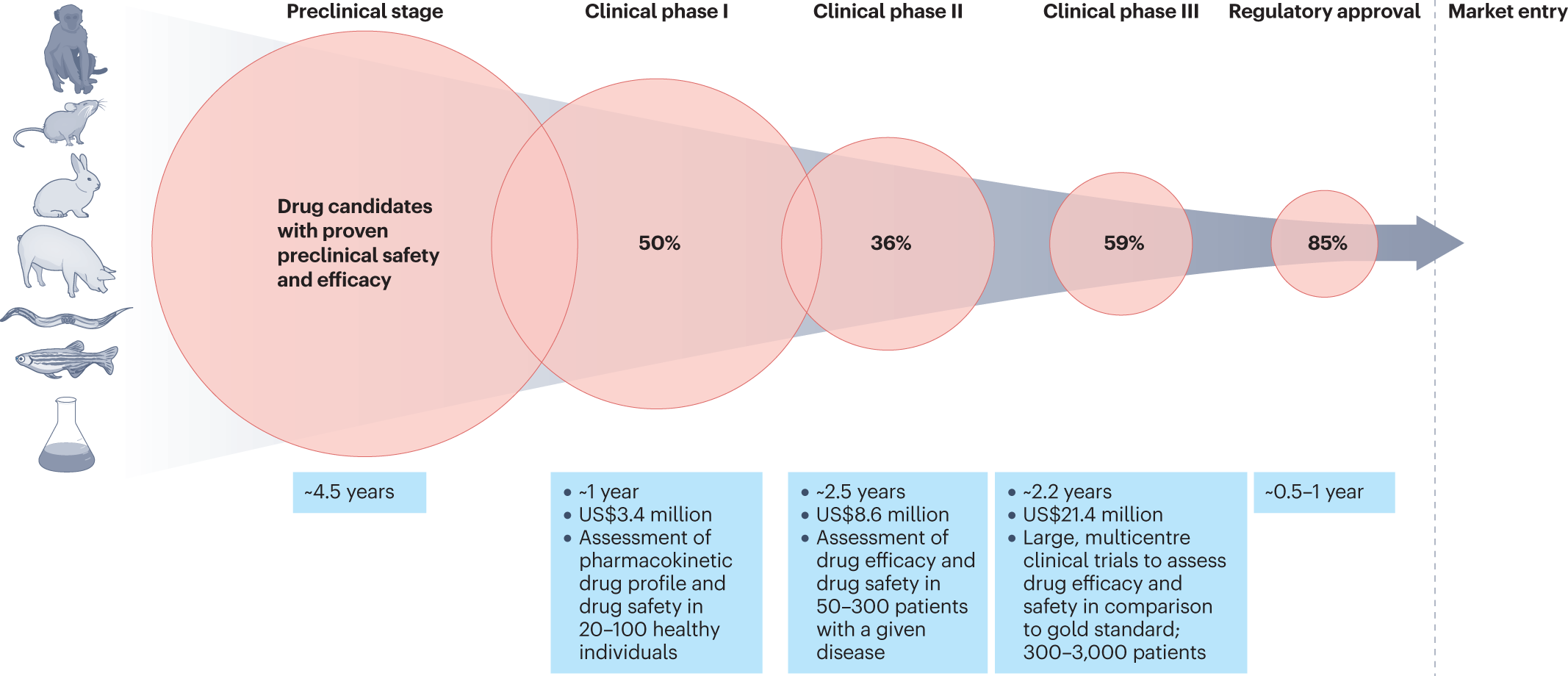
Human disease models in drug development
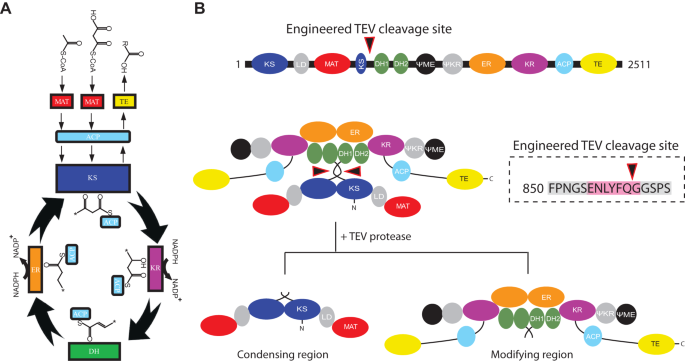
Atomic model for core modifying region of human fatty acid synthase in complex with Denifanstat
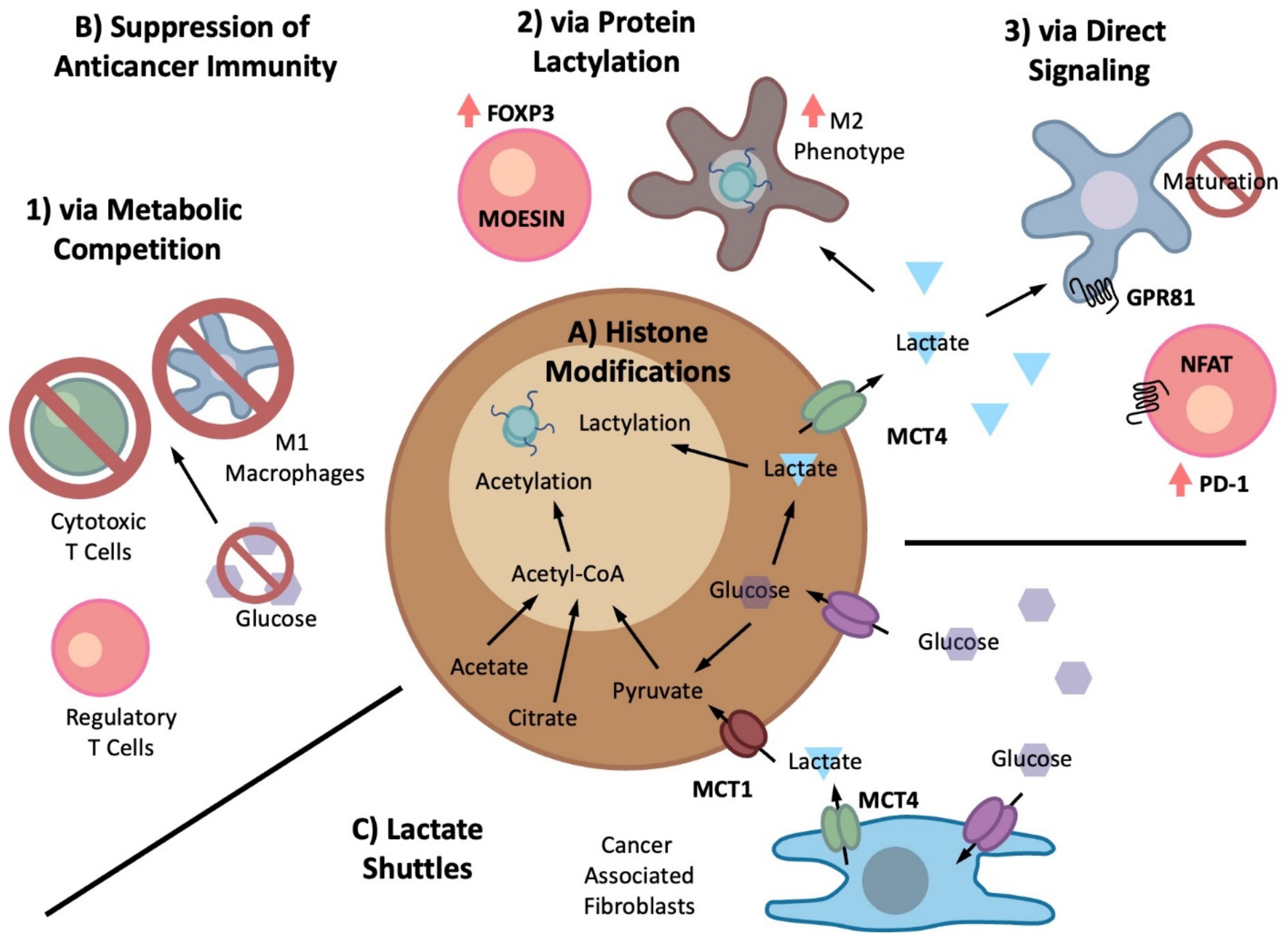
Biomolecules, Free Full-Text
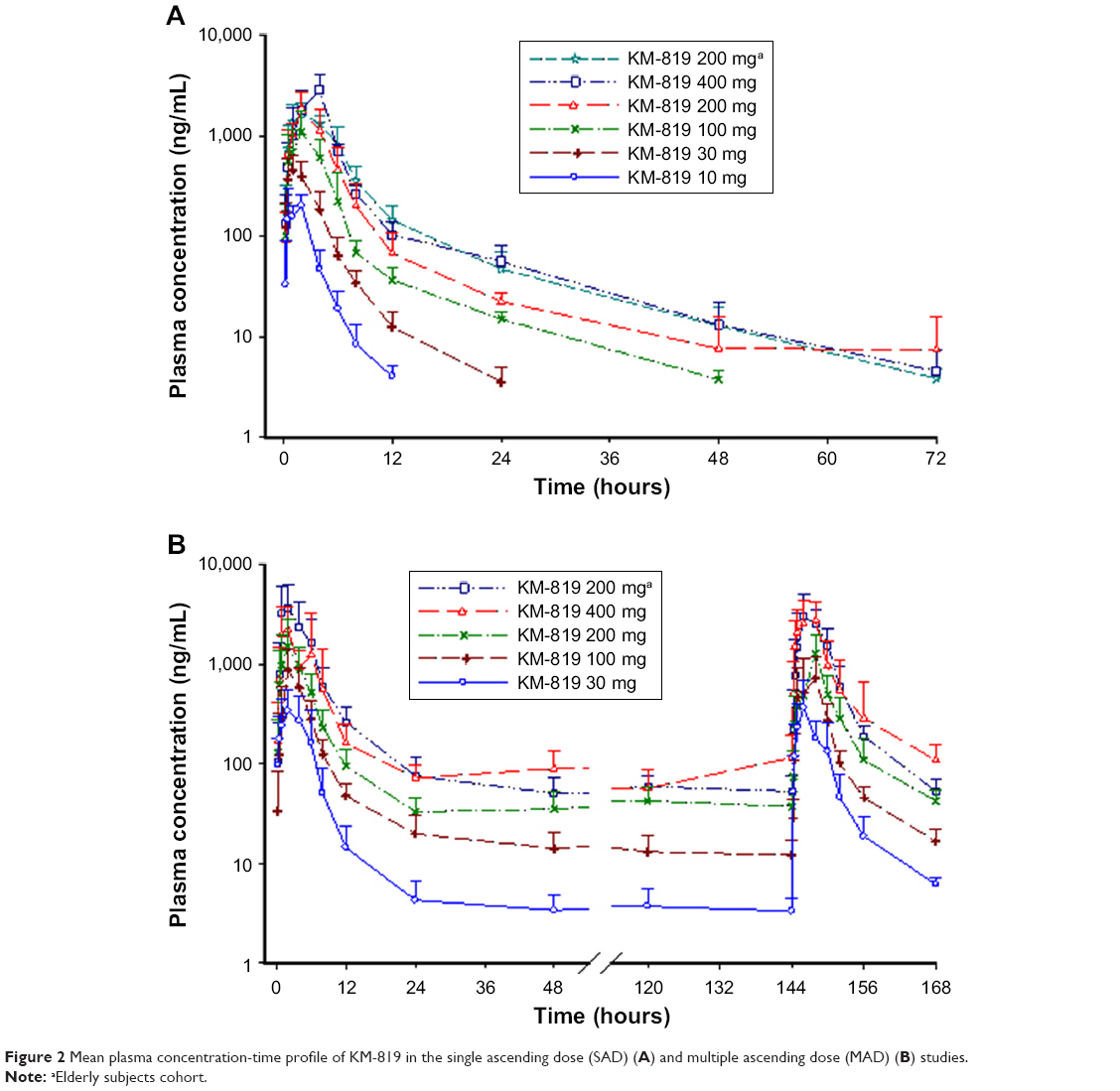
A first-in-human study to investigate the safety, tolerability

Safety, pharmacokinetics, and antitumour activity of trastuzumab

Early Safety Assessment - Drug Discovery and Development Based on
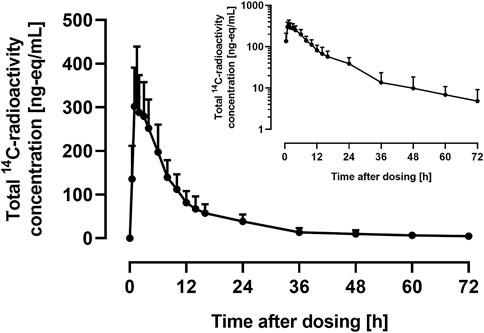
Frontiers Absorption, Metabolism, and Excretion of ACT-1004-1239