2.t 300 K, 36 g of glucose present per litre in itssolution has an
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would be itsconcentration?(1) 11 gl 1(3) 36 gl 1(2) 22 gL 1(4) 42 gL 1
2-t 300 K- 36 g of glucose present per litre in itssolution has an osmotic pressure of 4-98 bar- If theosmotic pressure of solution is 1-52 bar at thesame temperature- what would be itsconcentration-1- 11 gl-1-3- 36 gl-1-2- 22 gL-1-4- 42 gL-1

SOLUTION: class 12 chemistry quick revision notes - Studypool

WICUS). GUJ-CET ld be the osmotic pressure of the system 300 k temperature ? (R=8.314 x 10-2 1 k-1) (Assume that lomic solid substances completely dissociates in an aqueous 7. What would

2) 35.0 II. (1) 22.4 lit. (3) 448 lit. (4) 44800 ml Toleo At S.T.P., the density of nitrogen monoxide is - (2) 30 GL-1 (1)3.0 GL-1 (3) 1.34 gl-1 (4) 2.68 gL-1
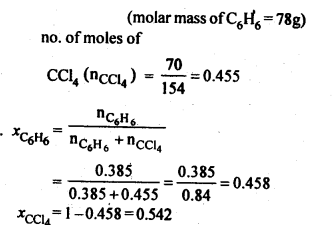
NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions

At 300 K, 30 g of glucose present in a litreof its solution has an osmotic pressure of4.98 bar--.
At 300 K, 36 g of glucose present in a litre of its solutio - Sarthaks eConnect

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions - CBSE Tuts

is U. 2 If solubility of gas X (1) 0.5 gl. lity of gas 'X' is 0.5 gl- 1 bar then its solubility 3 bar pressure will be (2) 1.5 GL- (3)