At 300 K, 36 g of glucose present per litre in its solution has an osm

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

At 300 K, 36 gof glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the same
EXP1 BIO560.docx - EXPERIMENT 1: FUNDAMENTAL PHYSIOLOGICAL PRINCIPLES POST LAB QUESTIONS A. Units of Measurement a. provide the correct conversion units

S2 Physiology Unit 2 - Body Fluid Physiology Flashcards
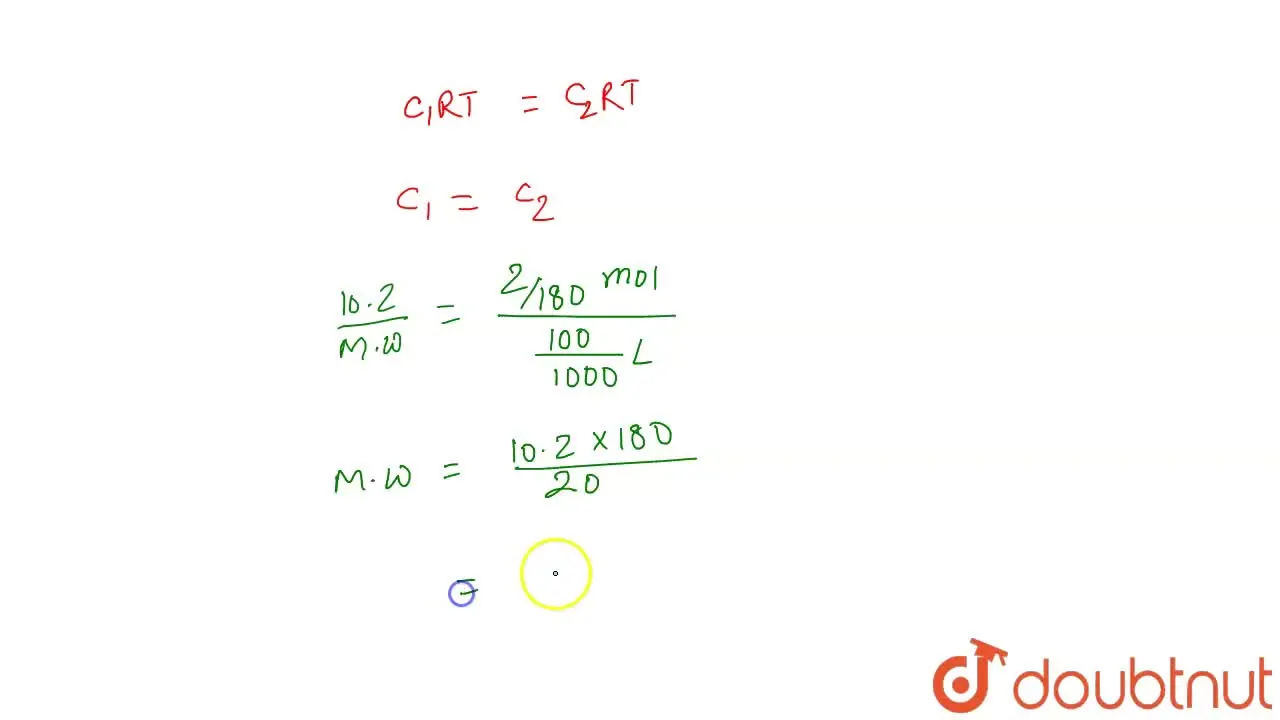
A solution containing 10.2 g of glycrine per litre is found to be isot

At `300 K`, `36 g` of glucose present per litre in its solution has an osmotic pressure of `4.98

ANSWERED] At 300 K 36 g of glucose present in a litre of its solution - Kunduz
Chapter 13.5: Colligative Properties - Chemistry LibreTexts

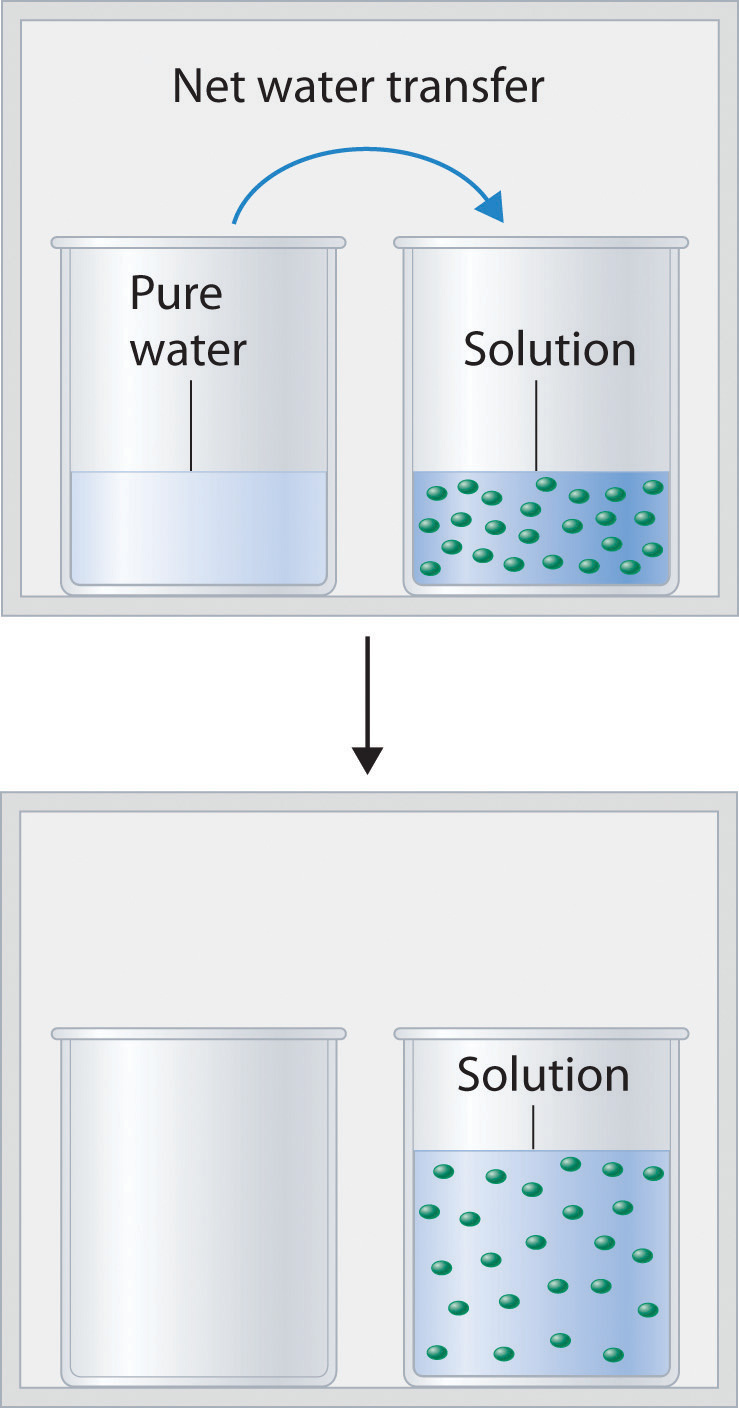
Unit 9: Critical care Analytes and Electrolytes & water balance
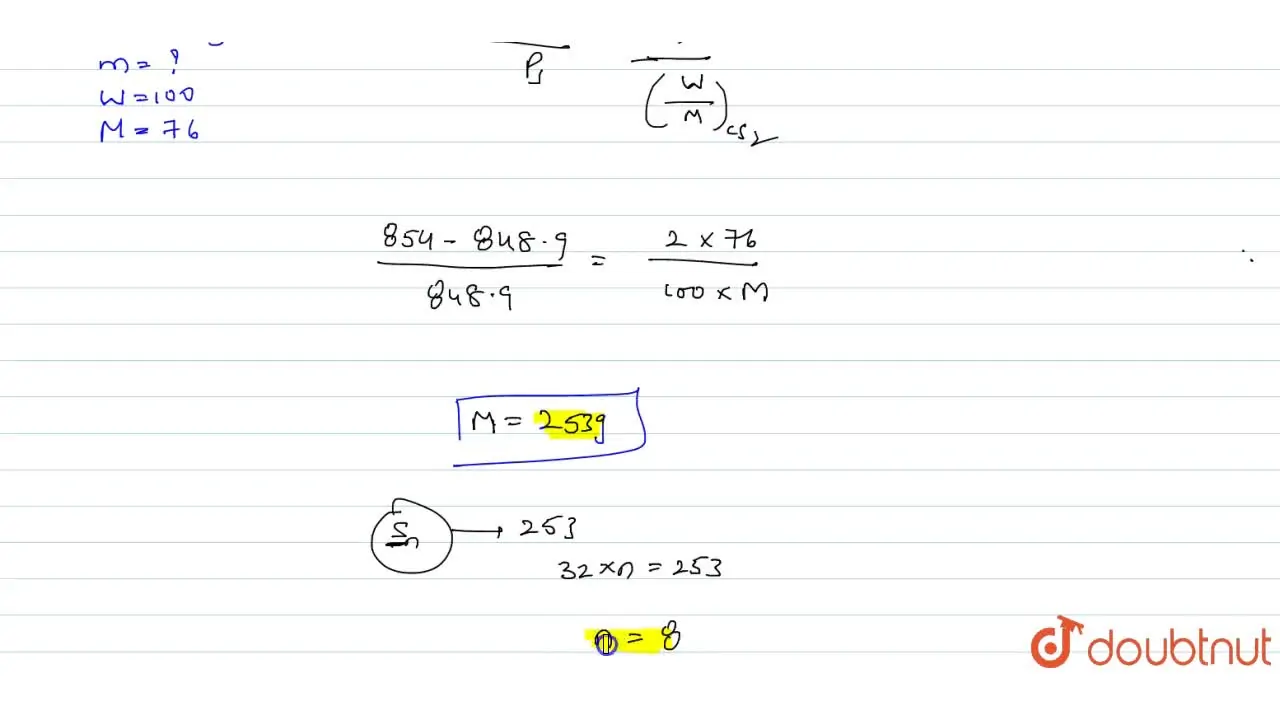
The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o