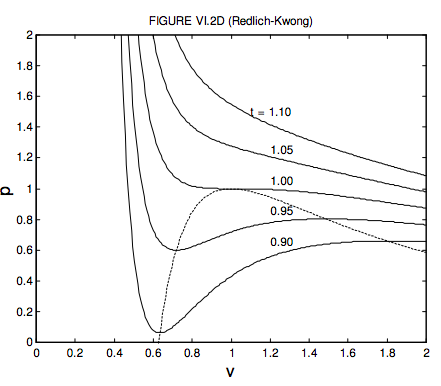
The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement

6.3: Van der Waals and Other Gases - Physics LibreTexts

The given graph in Fig represents the variation of Z compressibility f actor = pV / nRT vs.P, for the three real gases A , B and C.identify the only incorrect statement

Yucation The given graph represent the variations of Z (compressibility factor = pV) v/s p three nRT real gases, A, B and C. Identify the incorrect statement. p(atm) - A. For the

Yucation The given graph represent the variations of Z (compressibility factor = pV) v/s p three nRT real gases, A, B and C. Identify the incorrect statement. p(atm) - A. For the

The given graph represents the variations in compressibility factor (z) = pV /nRT versus p, for three real gases A, B and C.Which of the following statements is/are correct?a)For gas A, a =

Compressibility factor - Wikipedia
Where is the deviation of a gas from ideal behaviour maximum at? - Quora

From the given plot between Z and P , which of the following statements are correct for a real gas?

the given graph represents the variation of Z (compressibility factor =dfrac{PV}{nRT}) versus P, three real gases A, B and C. Identify the only correct statement.For the gas A, a = 0 and

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect

Non-Ideal Gas Behavior Chemistry: Atoms First
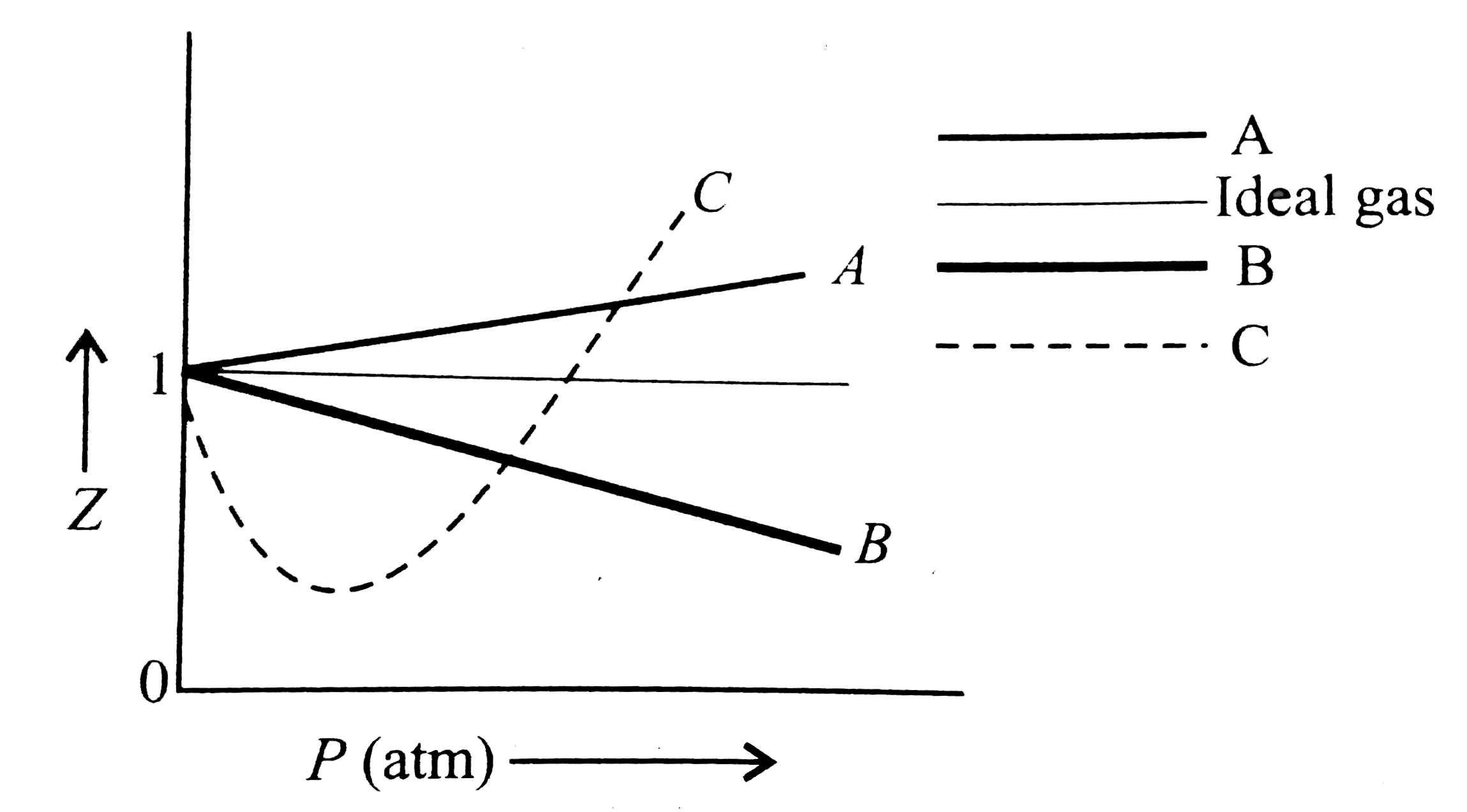
For gas C, which is a typical real gas, neither a nor b=0. By knowing

Determine Compressibility of Gases