Health-related quality of life and quality-adjusted progression
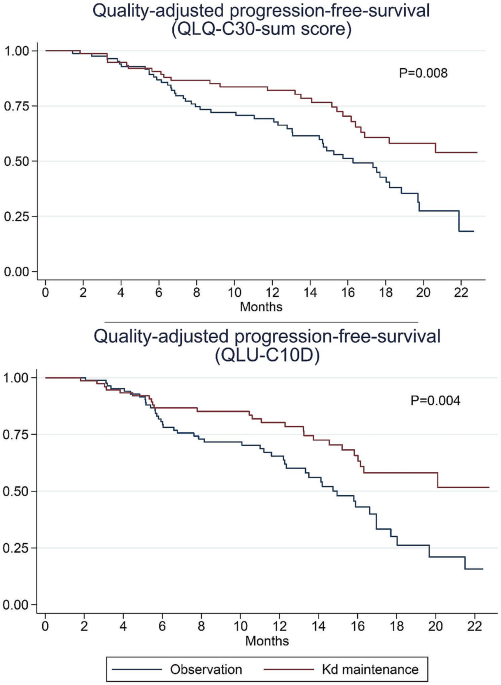
Background Decisions regarding maintenance therapy in patients with multiple myeloma should be based on both treatment efficacy and health-related quality of life (HRQL) consequences. In the CARFI trial, patients with first relapse of multiple myeloma underwent salvage autologous stem cell transplantation (salvage ASCT) before randomization to carfilzomib-dexamethasone maintenance therapy (Kd) or observation. The primary clinical endpoint was time to progression, which was extended by 8 months by Kd. The aim of this paper is to present the all HRQL endpoints of the CARFI trial including the HRQL effect of Kd maintenance therapy relative to observation. The primary HRQL endpoint was assessed by EORTC QLQ-C30 Summary score (QLQ-C30-sum) at 8 months follow-up. A key secondary HRQL endpoint was quality-adjusted progression-free-survival (QAPFS). Methods HRQL was assessed with EORTC QLQ-C30, EORTC QLQ-MY20 and FACT/GOG-Ntx at randomization and every second month during follow-up. HRQL data were analyzed with linear mixed effect models until 8 months follow-up. QAPFS per individual was calculated by multiplying progression-free survival (PFS) by two quality-adjustment metrics, the QLQ-C30-sum and EORTC Quality of Life Utility Measure-Core 10 dimensions (QLU-C10D). The QAPFS per treatment group was estimated with the Kaplan-Meier method. P < 0.05 was used for statistical significance, and a between-group minimal important difference of 10 points was interpreted as clinically relevant for the QLQ-C30-sum. Results 168 patients were randomized. HRQL questionnaire compliance was 93%. For the QLQ-C30-sum, the difference of 4.62 points (95% confidence interval (CI) -8.9: -0.4, p = 0.032) was not clinically relevant. PFS was 19.3 months for the Kd maintenance group and 16.8 months for the observation group; difference = 2.5 months (95% CI 0.5; 4.5). QAPFS based on the QLQ-C30-sum for the Kd maintenance group was 18.0 months (95% CI 16.4; 19.6) and for the observation group 15.0 months (95% CI 13.5; 16.5); difference = 3.0 months (95% CI 0.8–5.3). QAPFS based on the QLU-C10D for the Kd maintenance group was 17.5 months (95% CI 15.9; 19.2) and 14.0 months (95% CI 12.4; 15.5) for the observation group; difference = 3.5 months (95% CI 1.1–5.9). Conclusions Kd maintenance therapy after salvage ASCT did not adversely affect overall HRQL, but adjustment for HRQL reduced the PFS compared to unadjusted PFS. PFS of maintenance therapy should be quality-adjusted to balance the benefits and HRQL impact.
The quality-adjusted life-years in the oncological patients' health-related quality of life
Current Oncology, Free Full-Text

Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma
:max_bytes(150000):strip_icc()/TermDefinitions_PercapitaGDP-09e9332fe3d04e68b34e676554168077.jpg)
GDP Per Capita: Definition, Uses, and Highest Per Country

Ensure healthy lives and promote well-being for all at all ages

PDF) Health-related quality of life questionnaire for polycystic ovary syndrome (PCOSQ-50): development and psychometric properties

OUH - Group members

Association between racial discrimination and health-related

Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma
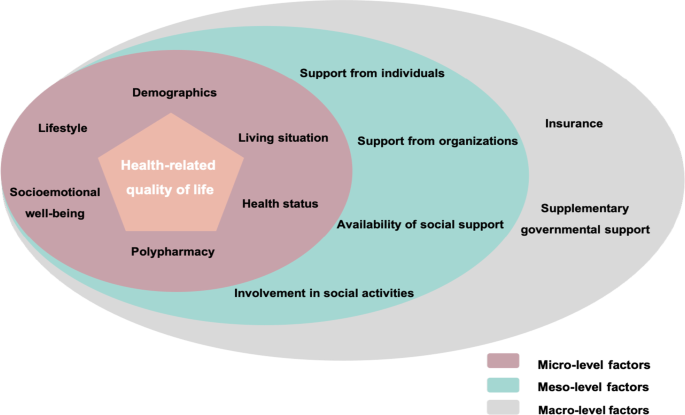
Factors associated with health-related quality of life among home-dwelling older adults aged 75 or older in Switzerland: a cross-sectional study, Health and Quality of Life Outcomes

Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma

Sören Möller — University of Southern Denmark

Basic principle of the quality-adjusted life year (QALY) in economic
:max_bytes(150000):strip_icc()/randd.asp-fb436cb94bed4c8a8032f732887153db.jpg)
Research and Development (R&D) Definition, Types, and Importance
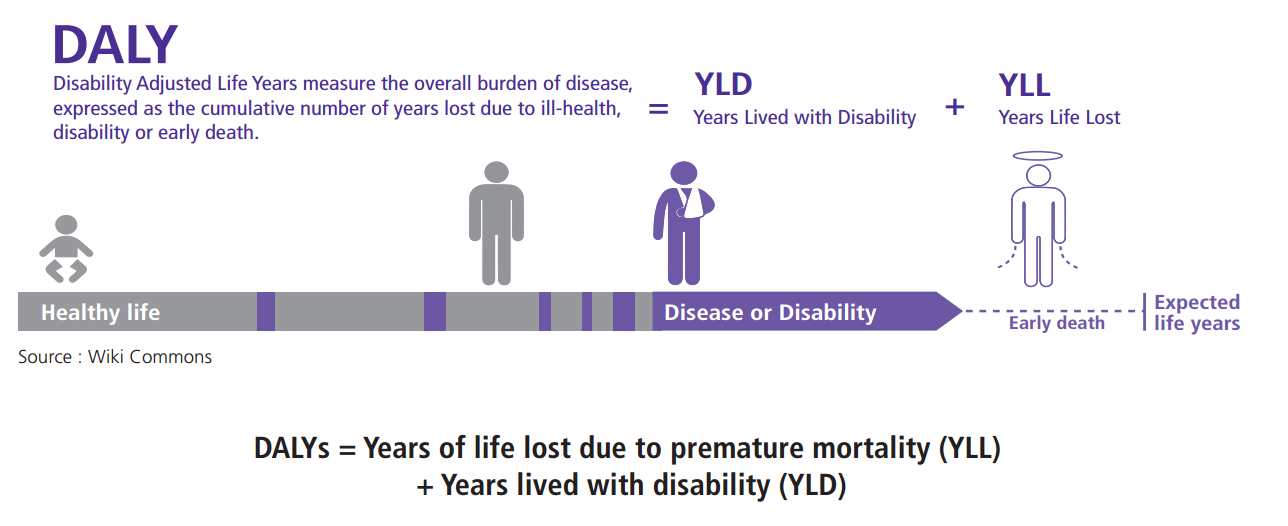
Understanding Summary Measures Used to Estimate the Burden of Disease: All about HALYs, DALYs and QALYs – National Collaborating Centre for Infectious Diseases