organic chemistry - Why is this diagram depicting the molecular orbital (MO) basis for a back-side attack the way it is? - Chemistry Stack Exchange

Consider: The description of this image in my textbook is as follows: In order to form a bond, the HOMO (the highest occupied molecular orbital) of one species must interact with the LUMO (the lo

Non-covalent interactions from a Quantum Chemical Topology perspective
OpenKIM · Morse Shifted GirifalcoWeizer 1959MedCutoff Fe MO_984358344196_004 MO_984358344196 · Interatomic Potentials and Force Fields
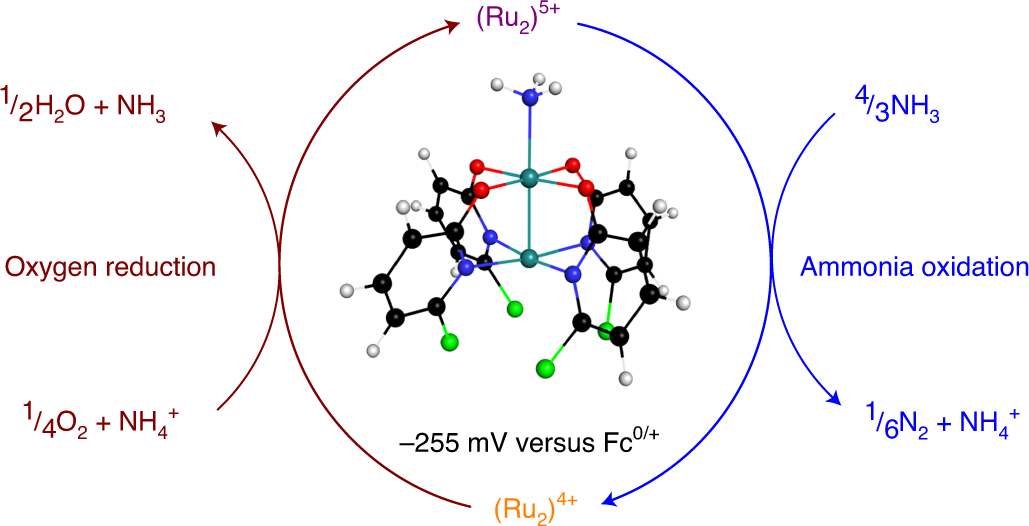
Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia
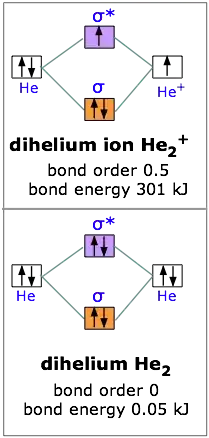
Molecular orbital theory & predicting the stability of a molecule? - Chemistry Stack Exchange

1.4 Molecular Orbital Theory

organic chemistry - When is donation into an anti-bonding MO stabilising? - Chemistry Stack Exchange

Advanced organic chemistry [Part A] 2006939782, 9780387448978, 9780387683461, 9780387448993

Present State of the Art and Future Challenges in the Hydrodesulfurization of Polyaromatic Sulfur Compounds - ScienceDirect
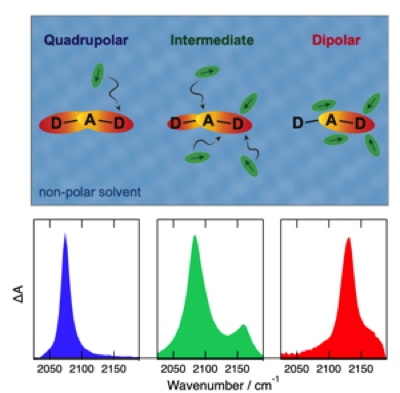
Université de Genève - Groupe du Professeur Andreas Hauser
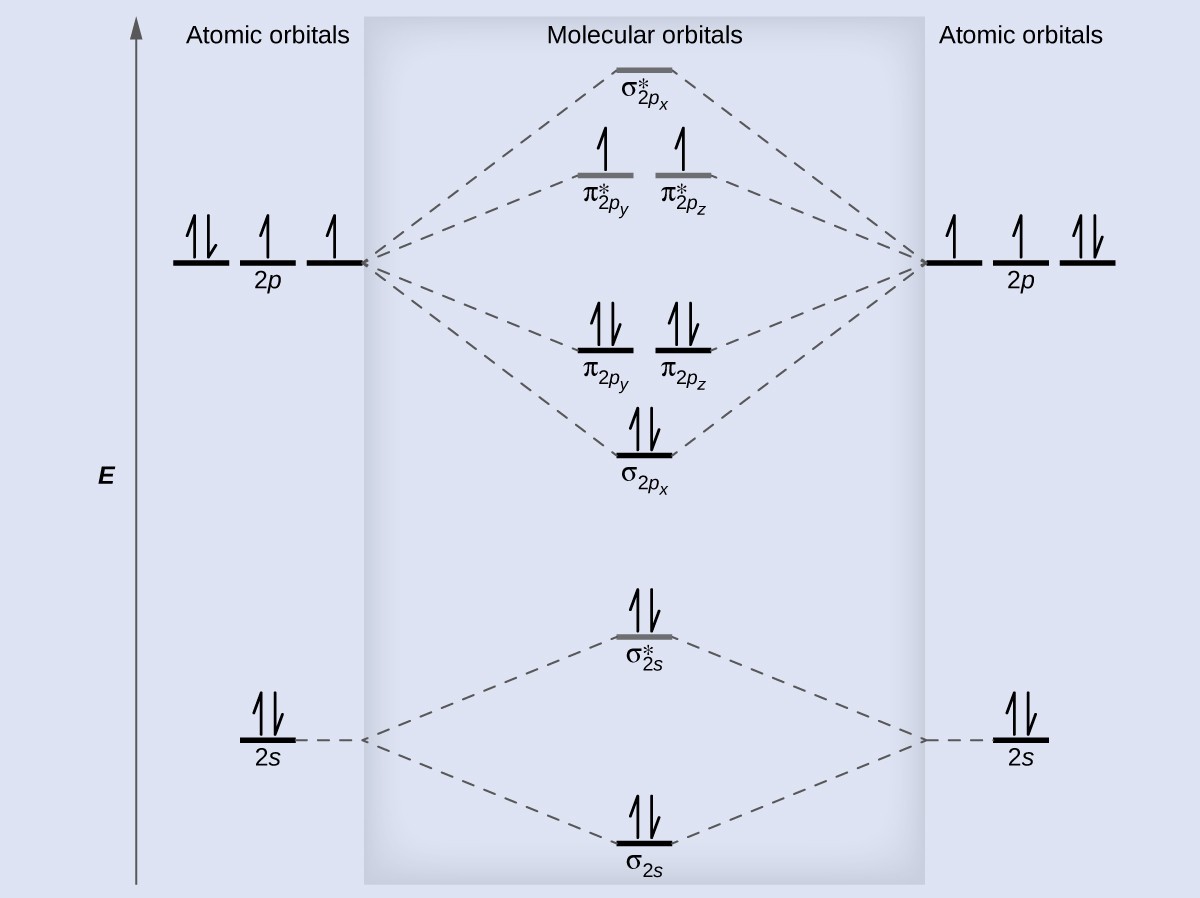
7.7 Molecular Orbital Theory – Chemistry Fundamentals

Chemistry, Free Full-Text