An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics

An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most.
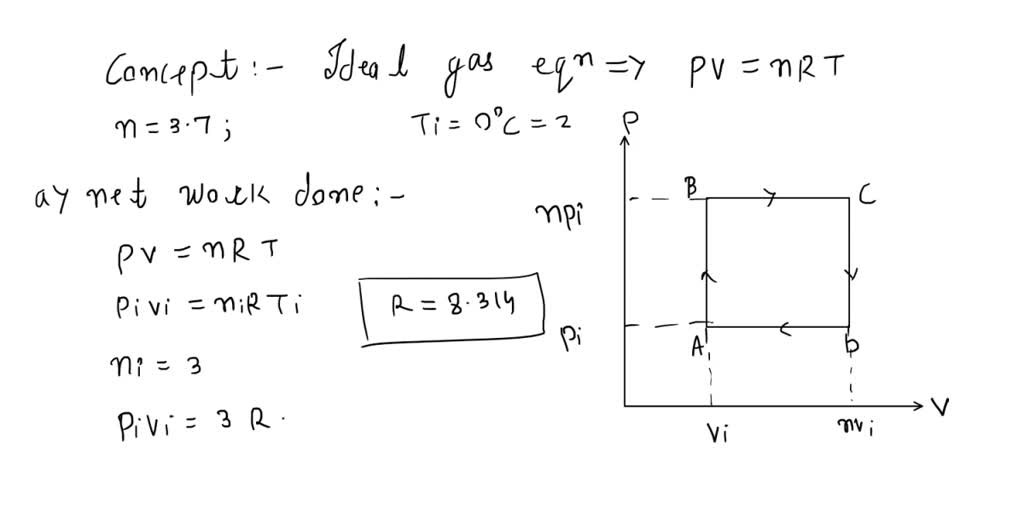
SOLVED: ideal gas initially at Pi, Vi, and Ti is taken through cycle as shown below: (Let the factor n 3.7.) nf Find the net work done on the gas per cycle

Heat Engines and Efficiency - ppt download

Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0562-0612), PDF, Heat
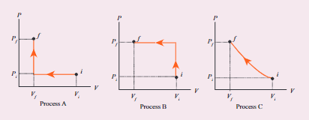
An ideal gas is taken fromPiVitoPfVfin three different ways Identify the process in which the work done on the gas the most

Graphically show the total work done in an expansion when the state of an ideal gas is changed reversibly and isothermally from pi, Vi to pf, Vf With the help of p

Thermodynamic Principal #chemical engineering microproject

Best Practice Examples

P) Thermodynamics, PDF, Gases

PPT - Gases, Heat, and Work PowerPoint Presentation, free download - ID:5076115

Thermodynamics - Physics at Oregon State University

Heat & Thermodynamics [3 ed.]