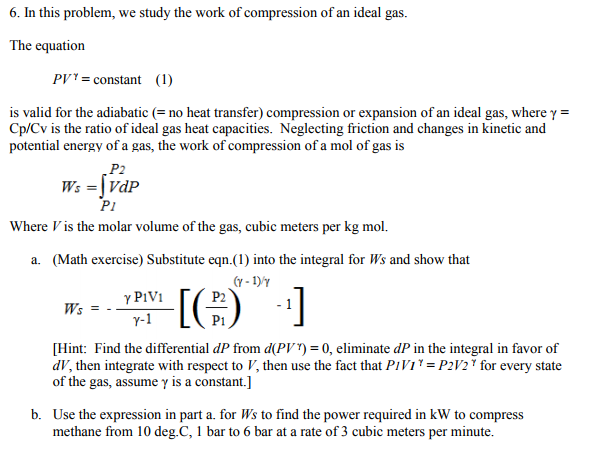
Solved ion of an ideal gas. The equation PI = constant (1)
How I find the a and b constant in the Van der Waals equation? - Quora

SOLVED: ) One mole of an ideal gas expands from 5 to 1 bar at 298 K. Calculate w (a) for a reversible expansion and (b) for an expansion against a constant

Gases.pptx
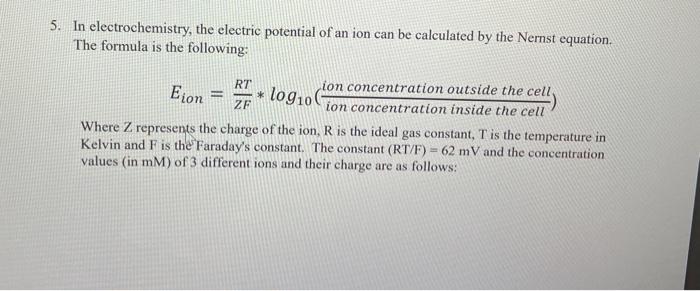
Solved In electrochemistry, the electric potential of an ion
How I find the a and b constant in the Van der Waals equation? - Quora

Using the Ideal Gas Equation: Example Problem #1
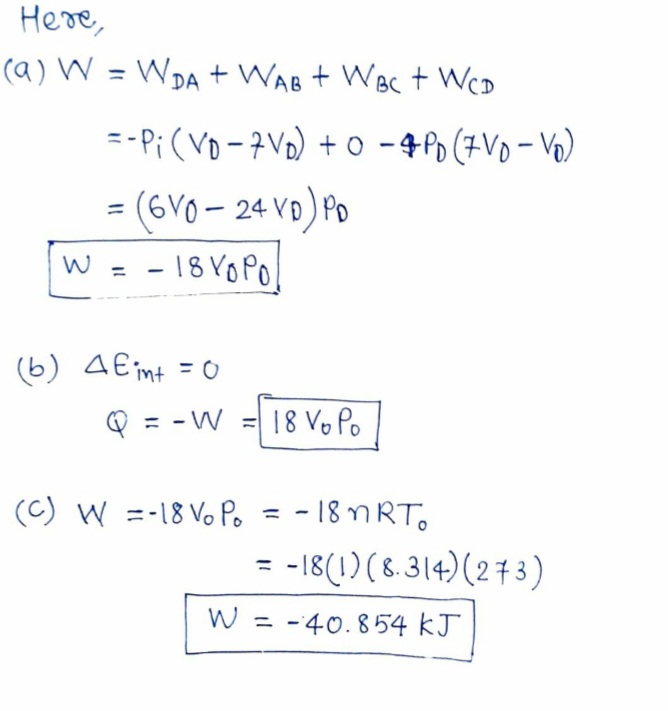
Answered: An ideal gas initially at pressure P0,…

Molar Mass & Ideal Gas Law, Overview, Formula & Examples - Lesson
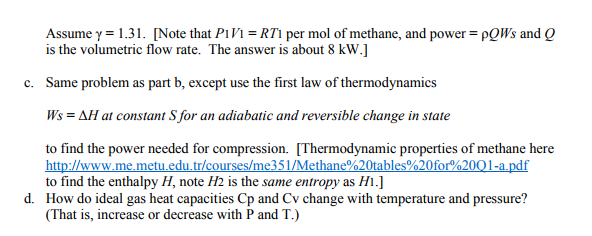
Solved ion of an ideal gas. The equation PI = constant (1)

Ideal Monatomic Gas - an overview