The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

At high pressure, the compressibility factor for one mole of van der w

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible, calculate the van der Waals' constant 'a' Domeik

Solved Show that the compressibility factor of van der Waals

Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an
Oxygen is present in one litre flask at a pressure of 7.6 x 10^-10 mm Hg. Calculate the number of oxygen molecules in the flask at 0°C. - Sarthaks eConnect

The compression factor (compressibility factor) for 1 mol of a van der
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
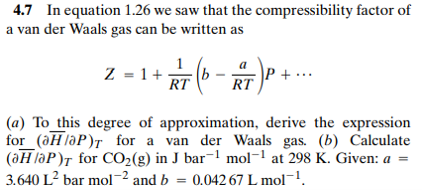
Solved 4.7 In equation 1.26 we saw that the compressibility

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
For 1 mol of gas, the plot of pV vs p is shown below. p is the pressure and V is the volume of the gas. - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0°C - Sarthaks eConnect

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}
The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT - Sarthaks eConnect