20.If Z is a compressibility factor, van der Waals equation at low
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

The compressibility factor (Z) of one mole of a van der Waals' gas

Solved Non-Ideal gases (mixed methods) 1. Use the
20.If Z is a compressibility factor, van der Waals equation at low

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor - Wikipedia

In the plot of Z (compressibility factor) vs P,Z attains a value of un

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

If Z is a compressibility factor, van der Waal's equation low pressure can be written as : tot gnolaszemit sem st263 nisho ad Phim shuplamenu Pb (1) Z = 1 - (
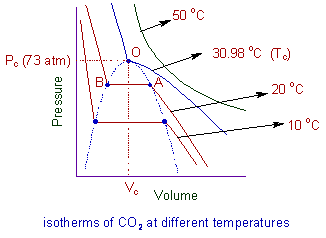
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR