physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Non-Ideal Gas Behavior Chemistry: Atoms First

Description of real gases: Compression factor
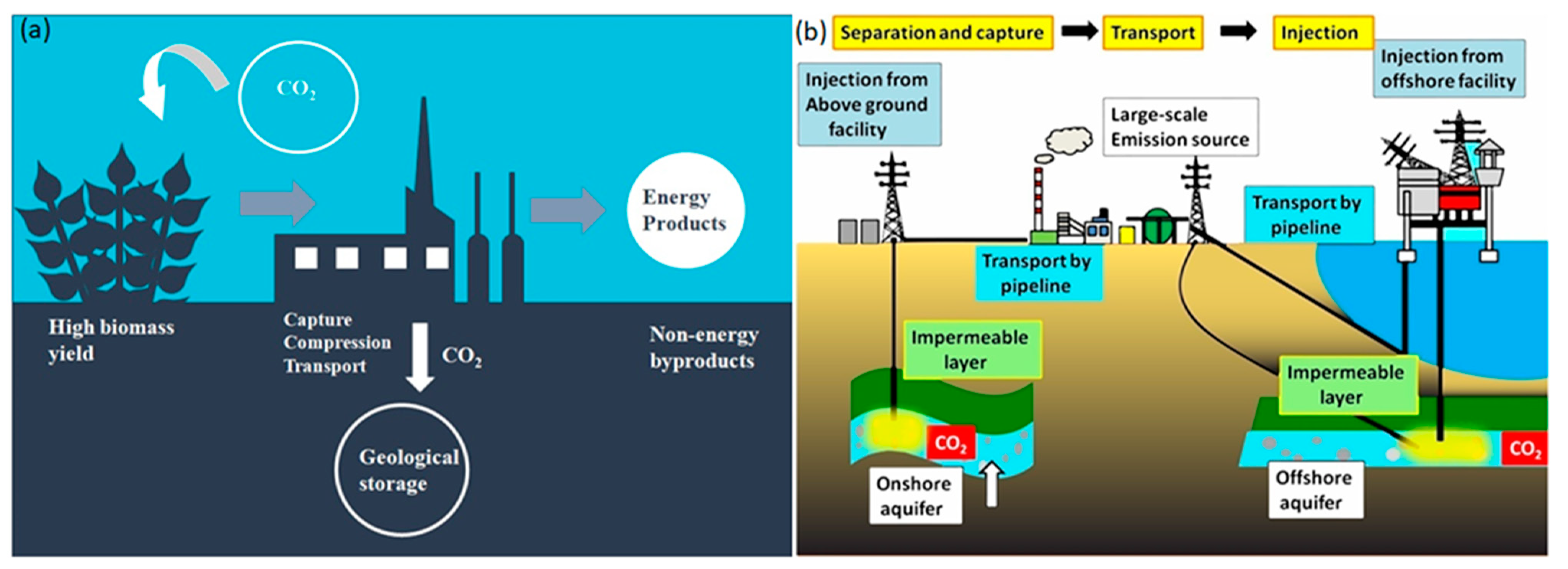
Energies, Free Full-Text

822 questions with answers in PHYSICAL CHEMISTRY
What is the maximum temperature stability of carbon fiber

Non-Newtonian Flow to the Theoretical Strength of Glasses via

physical chemistry - Why do some gases have lower value of Z for a

Energies, Free Full-Text
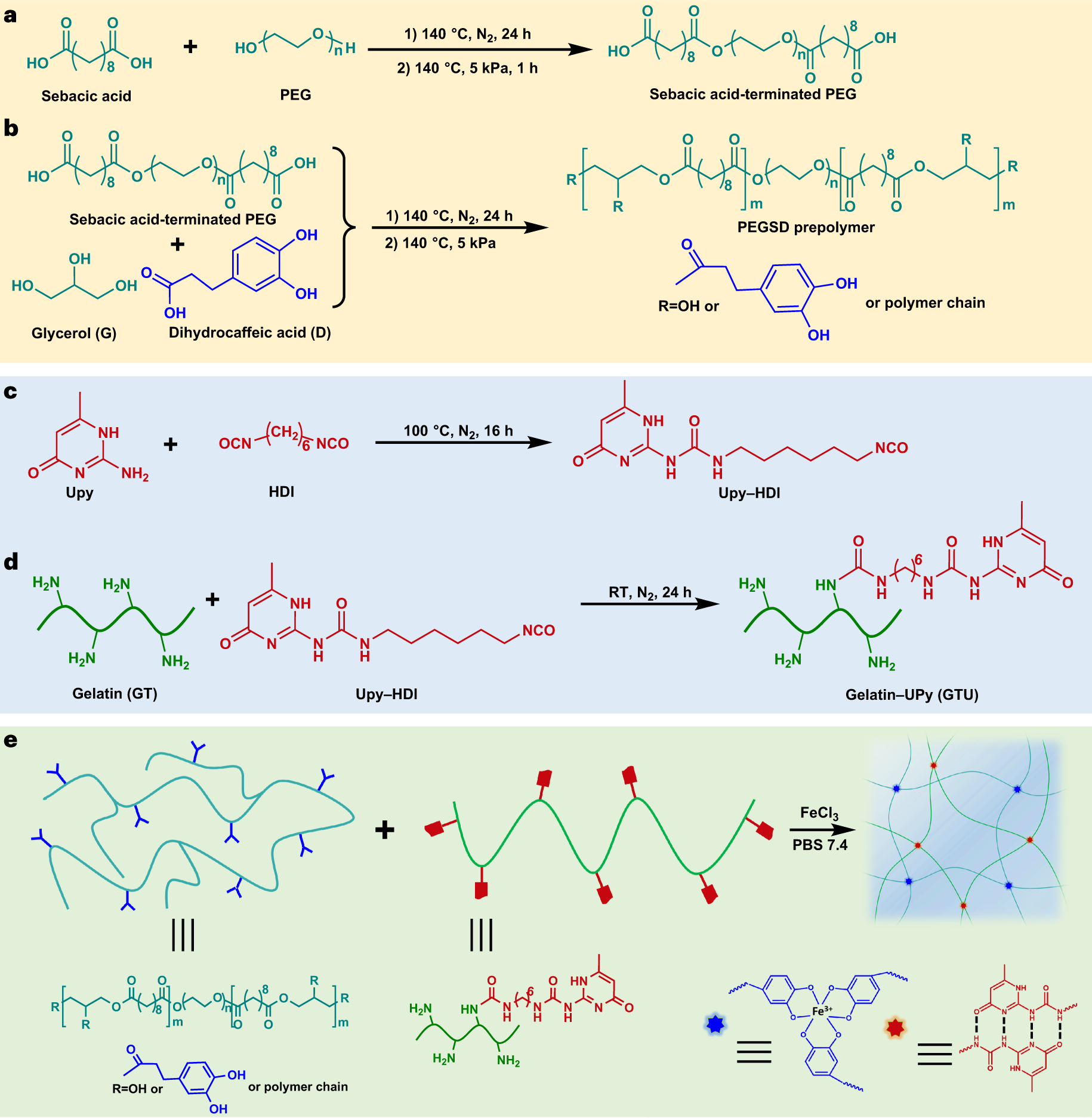
Physical dynamic double-network hydrogels as dressings to
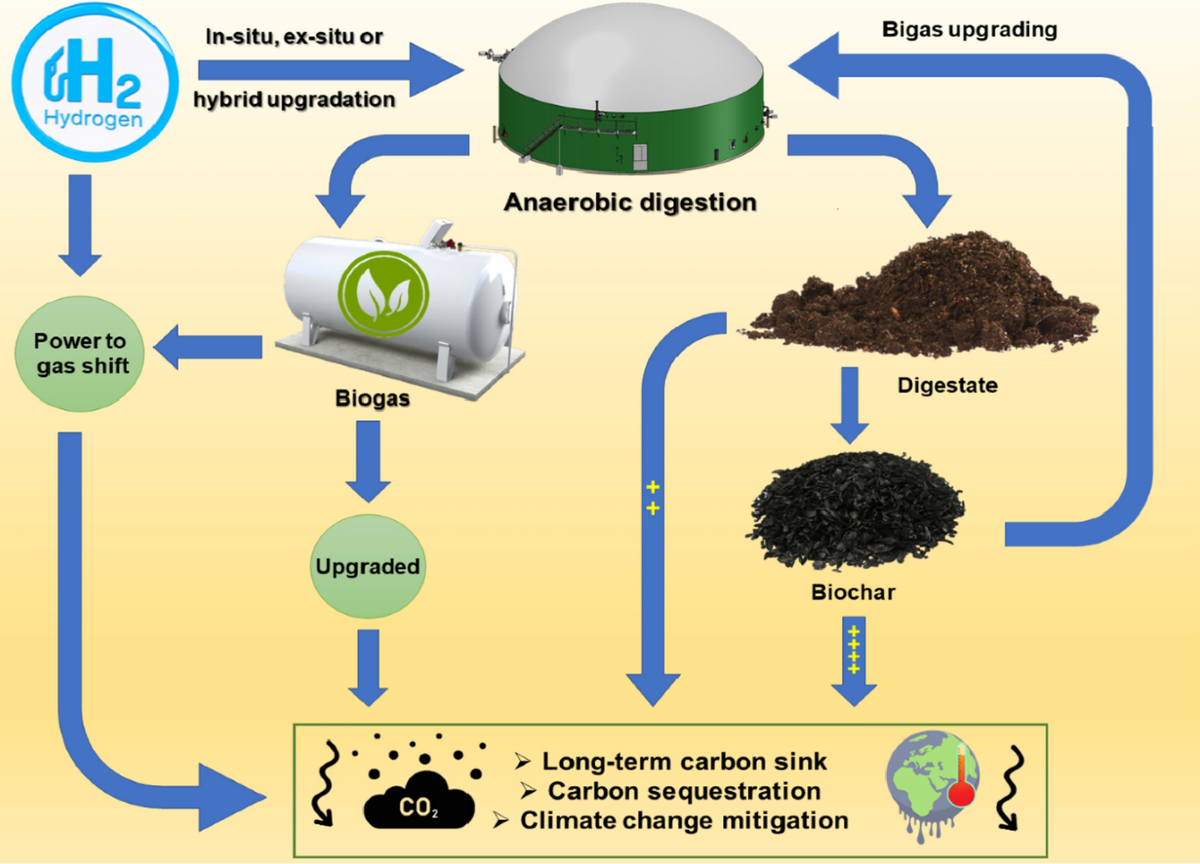
Integration of biogas systems into a carbon zero and hydrogen
Why does water compress more than other gases? - Quora