Solved The compressibility factor, Z, can be thought of as a

Answer to Solved The compressibility factor, Z, can be thought of as a

Gas Compressibility - an overview

Real Gas Behavior The Compression Factor (Z) [Example #2]

If compressibility factor Gas A, Gas B, Gas C and Gas D1.6,0.8,0.4,1.8 respectively than (i) Nature of gas (ii) Increasing order of force of attraction b/w the molecules (iii) which one gas

Select the correct statement : (a) The value of compressibility factor ' Z ' for H_2 gas is great
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Gas Compressibility Factor and Control Valve Sizing

Compressibility factor (gases) - Citizendium

For compressibility factor, Z, which of the following is /are correct?

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Compressibility Factor - an overview

1. The compressibility factor, z, is the ratio of

Compressibility Factor Z // Thermodynamics - Class 85
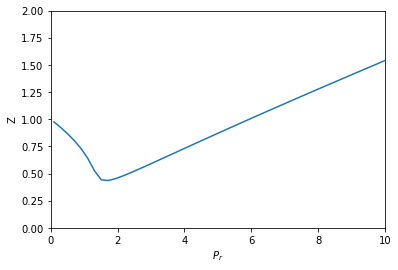
Compressibility factor variation from the van der Waals equation by three different approaches

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks