FDA has new mammogram guidelines for dense breast disclosure. What do the rules mean for Pennsylvania residents?
/cloudfront-us-east-1.images.arcpublishing.com/pmn/EMEDJI4AQVBZJNJHKLTY2ZSPJY.jpg)
In Pennsylvania, senators unanimously voted in favor of a bill to fund genetic testing to women at higher risk of breast cancer.
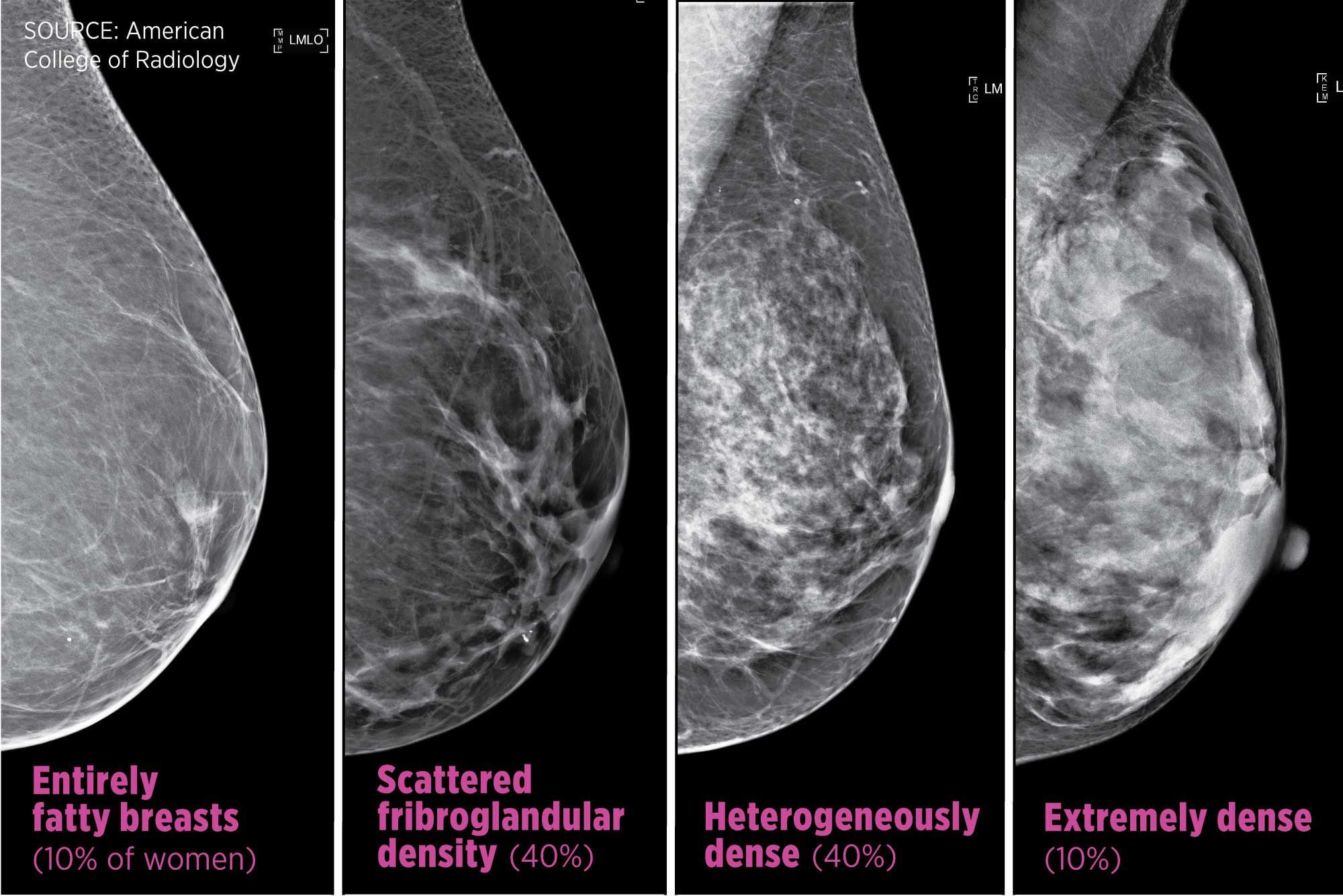
FDA has new mammogram guidelines for dense breast disclosure. What do the rules mean for Pennsylvania residents?

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News
NIH Mammogram Recommendations

Rad Tech CE, ASRT, ARRT® CE, Category A Credits
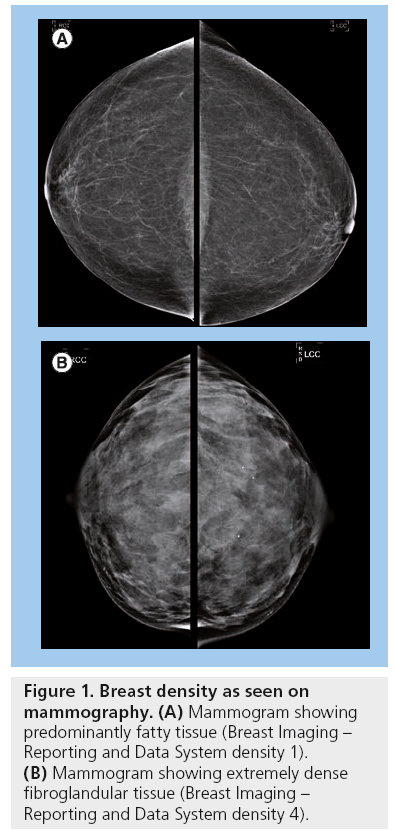
Automated breast ultrasound: a novel approach to screening women

Guidelines & Research — Operation Breast Density
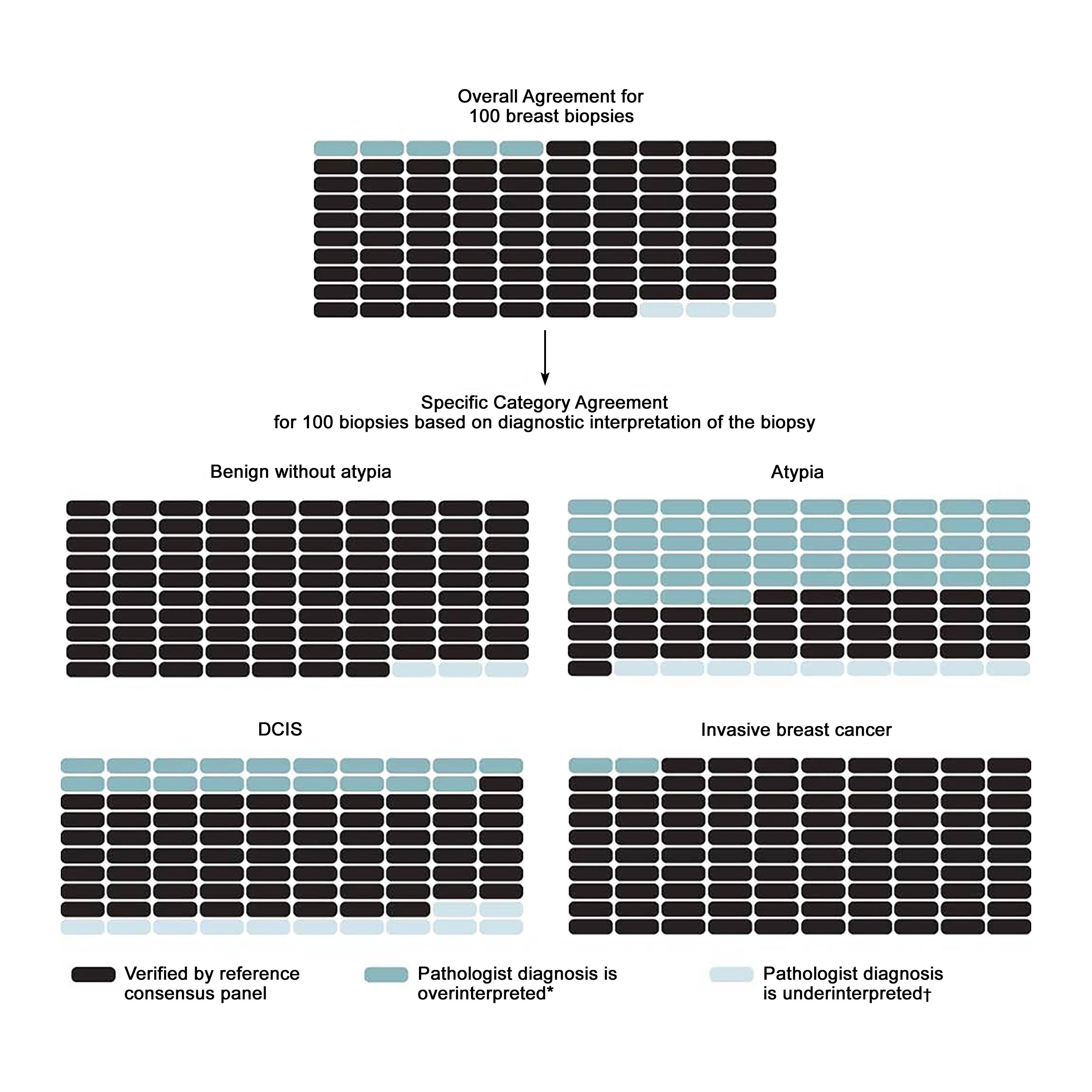
Breast Cancer Screening (PDQ®) - NCI

The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions

FDA rule change requires mammogram centers to notify patients of breast density

Imaging After Breast Cancer
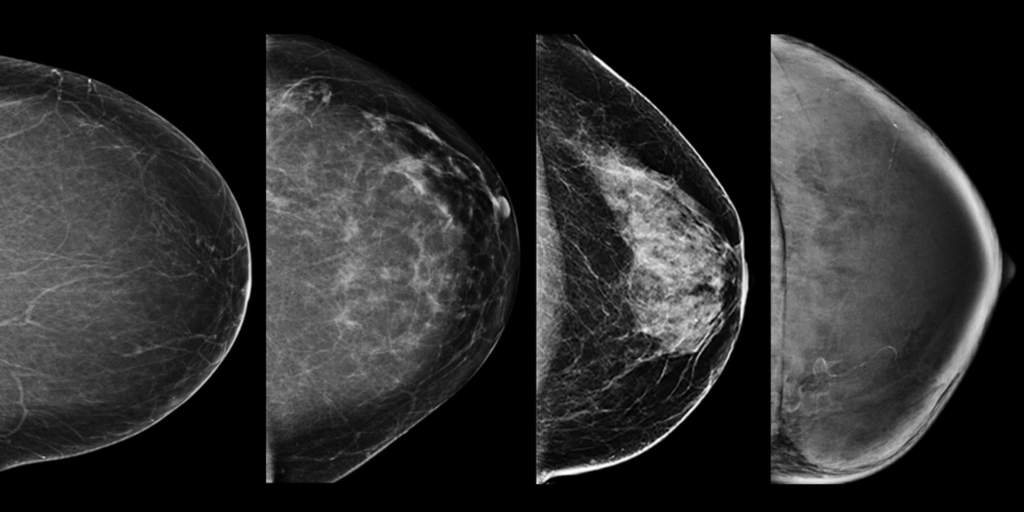
Breast density notification laws blanket 90% of U.S. women, yet still no national reporting standard is at hand. Why is that?