Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in

Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T 2. The final pressure Pf is :P i T 1 T 2/ T 1+ T 2B. 2 P i T 1/ T 1+ T 2C. 2 P i T 1 T 2/ T 1+ T 2D. 2 P i T 2/ T 1+ T 2
Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in the figure below- The temperature of one of the bulbs is then raised to T 2- The final pressure Pf is -P i T 1 T 2- T 1- T 2B- 2 P i T 1- T 1- T 2C- 2 P i T 1 T 2- T 1- T 2D- 2 P i T 2- T 1- T 2
The correct option is D 2P_i ( T_2T_1+T_2 )Since the above system is a closed one, the total number of moles of the ideal gas will be equal before and after th

Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure
Two flasks at the same temperature are joined by a glass tube with a stopcock. Flask A is a 4.0 L flask containing N2 (g) at 2.0 ATM, while flask B is
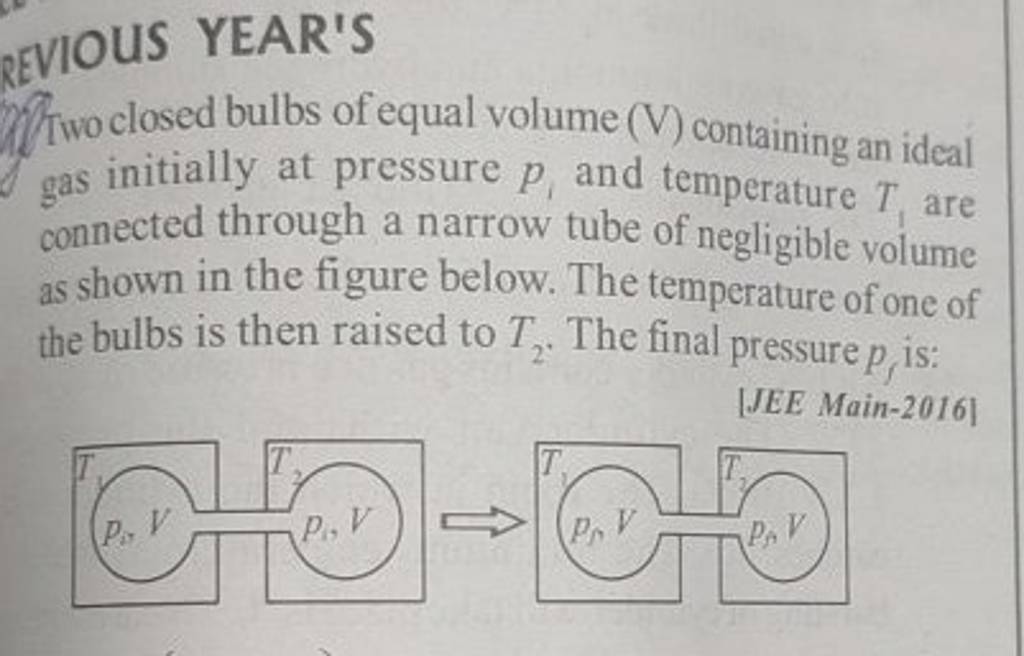
Two closed bulbs of equal volume ( V ) containing an ideal gas initially ..

Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in
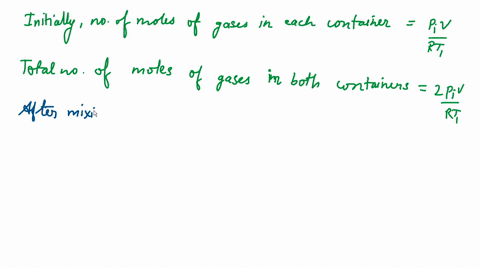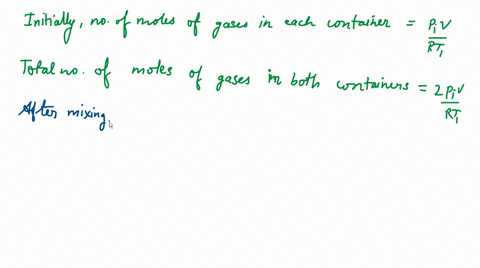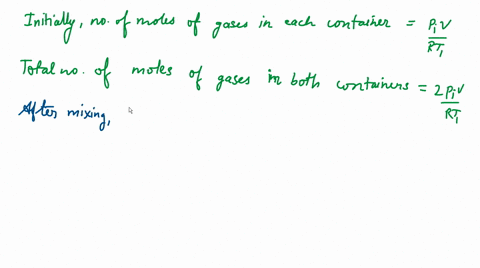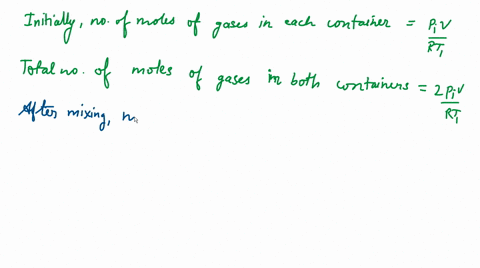
⏩SOLVED:Two closed bulbs of equal volume (V) containing an ideal gas…

Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure

Expansion of Gas, PDF, Thermal Expansion

The initial volume of a gas cylinder is 750.0 mL. If the pressure

Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in

Two closed bulbs of equal volume V containing an ideal gas initially at pressure p i and temperatureT 1 are connected through a narrow tube of negligible volume as shown in the
Ideal gas question on glass bulbs

Gaseous State - 2 Free MCQ Practice Test with Solutions - Chemistry
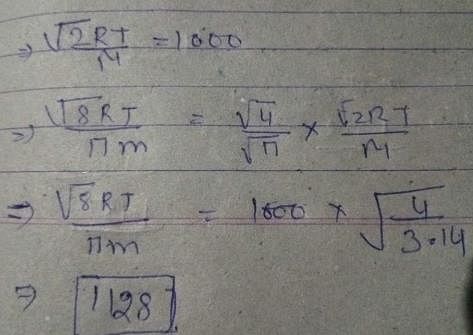
Gaseous State - 2 Free MCQ Practice Test with Solutions - Chemistry
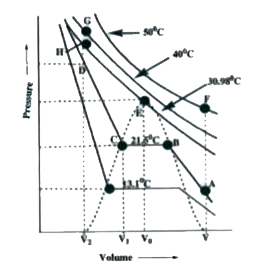
Telugu] Two closed vessel A and B of equal volume containing air at p