Solved What is the equilibrium constant (Kp) at 45 °C for

Answer to Solved What is the equilibrium constant (Kp) at 45 °C for

For reactions in the gas phase, an equilibrium constant may be written in terms of molarity (Kc) or in
search-static.byjusweb.com/question-images/aakash_
The equilibrium constant (K) for the reaction,2SO2(g)+O2(g)2S03(g) at 1000 K is 3.5 atmWhat would be the partial pressure of oxygen gas,if the equilibrium is found to have equal moles ofSO2 and SO3?

i.ytimg.com/vi/4dKCx2crbG8/maxresdefault.jpg
At 444^° C, the equilibrium constant K for the reaction 2AB gives A2 +B2,The degree of dissociation of AB will be (A) 10

Solved The equilibrium constant, Kp, has a value of 6.5×10−4

Equilibrium constant K(p) for the reaction CaCO(3)(s) hArr CaO(s) + CO

How to Calculate the Equilibrium Constant, K

Consider the reaction: 2 NO( g) + O2( g) ∆ 2 NO2( g) The followin

Calculating equilibrium constant Kp using partial pressures (article)

Solved Question 5 A reaction has an equilibrium constant of

Simple new correlation for the prediction of equilibrium constant (KP) of Haber reaction covering the industrial conditions - ScienceDirect

Chapter 14
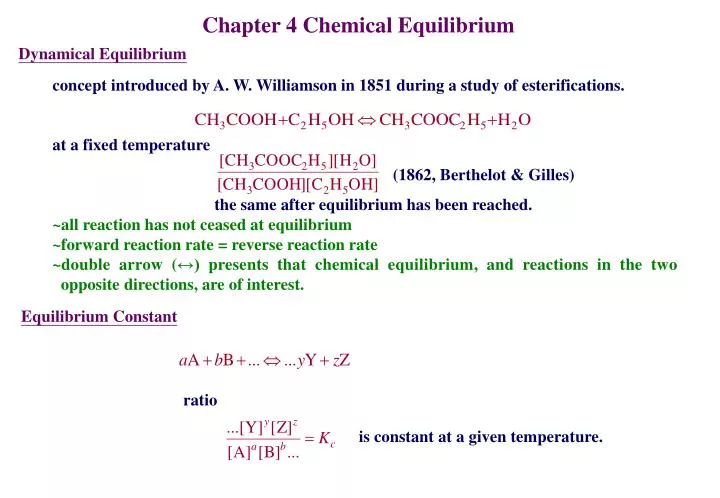
PPT - Chapter 4 Chemical Equilibrium PowerPoint Presentation, free download - ID:6955543
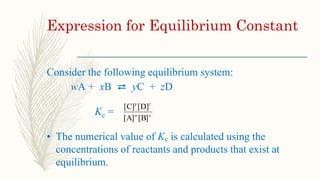
image.slidesharecdn.com/equilibrium-constant-prese