Registry Data Show Vedolizumab, Ustekinumab Safe in Pregnancy


Efficacy of ustekinumab, vedolizumab, or a second anti-TNF agent after the failure of a first anti-TNF agent in patients with Crohn's disease: a multicentre retrospective study, BMC Gastroenterology

Full article: Prospective observational study on Stelara (ustekinumab) assessing effectiveness in Crohn's disease (PROSE): a 16-week follow-up

IBD in Pregnancy: Ustekinumab, Vedolizumab Use Appears Safe
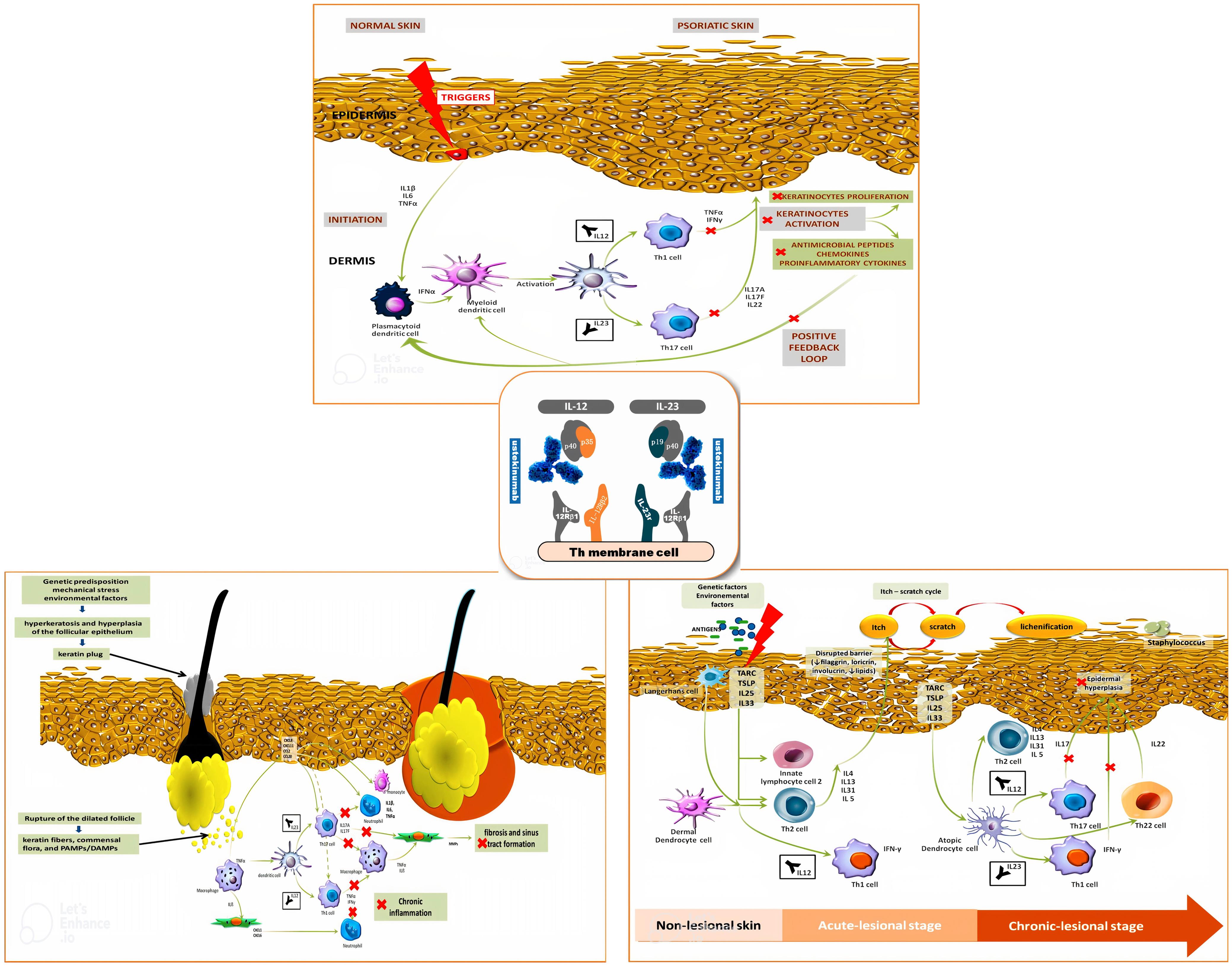
Ustekinumab in Dermatology: Approved Indications and Off-label Uses

Vincent S. Panella on LinkedIn: Registry-Data-Show-Vedolizumab-Ustekinumab- Safe-in-Pregnancy
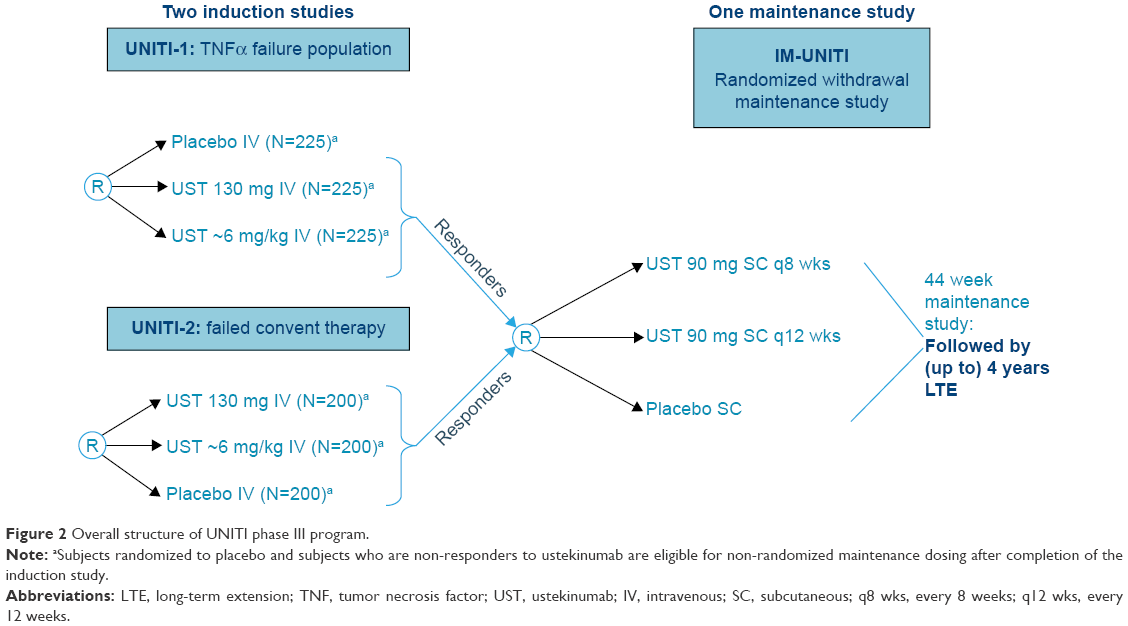
Ustekinumab in treatment of Crohn's disease: design, developmen

Ustekinumab Is Associated with Real-World Long-Term Effectiveness and Improved Health-Related Quality of Life in Crohn's Disease

About IBD Podcast Episode 92 - IBD and Pregnancy With Jill Gaidos, MD

A review article of inflammatory bowel disease treatment and pharmacogenomics, Beni-Suef University Journal of Basic and Applied Sciences

Use of vedolizumab throughout pregnancy. Trimester 1: exclusion of 12
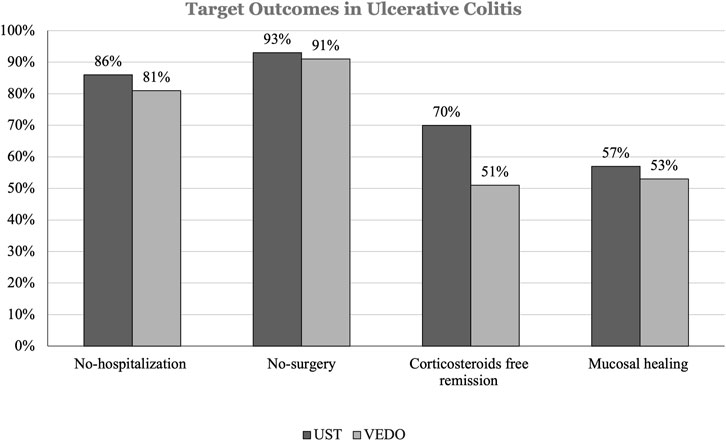
Frontiers Effectiveness of vedolizumab and ustekinumab as second biologic agent in achieving target outcomes in tumor necrosis factor antagonists experienced patients with inflammatory bowel disease (enroll-ex study)
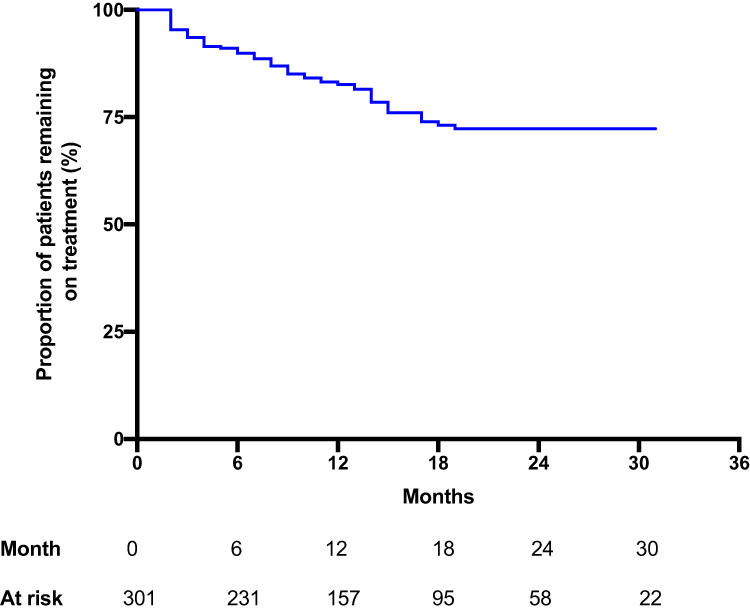
Treatment Persistence Of Ustekinumab In Crohn's Disease
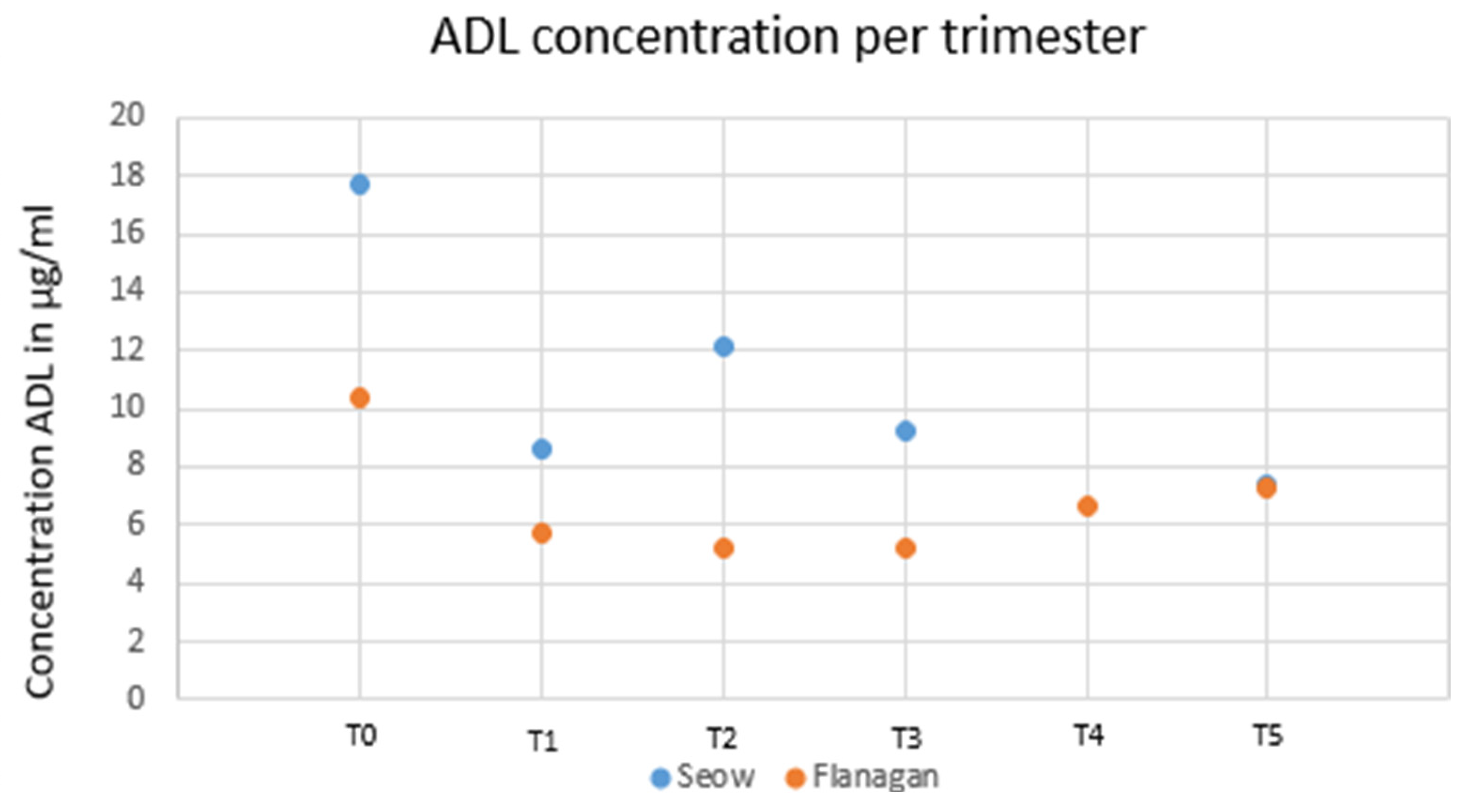
JCM, Free Full-Text

Ustekinumab is associated with superior treatment persistence but not with higher remission rates versus vedolizumab in patients with refractory Crohn's disease: results from a multicentre cohort study - Péter Bacsur, Mária Matuz

PDF) Ustekinumab Drug Levels in Maternal and Cord Blood in a Woman With Crohn's Disease Treated Until 33 Weeks of Gestation