If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
If Z is a compressibility factor, van der Waals

Radical Occupation, Radical Spatiality. Unconference. Think Space., PDF

If Z is compressibility factor, vander Waals equation low pressure can be written as

At low pressures For 1 mole, the van der Waals equation is written as [ p + a / V 2] V = RT The compressibility factor is then equal to:A. 1
If Z is a compressibility factor, van der Waals equation at low pressure ..
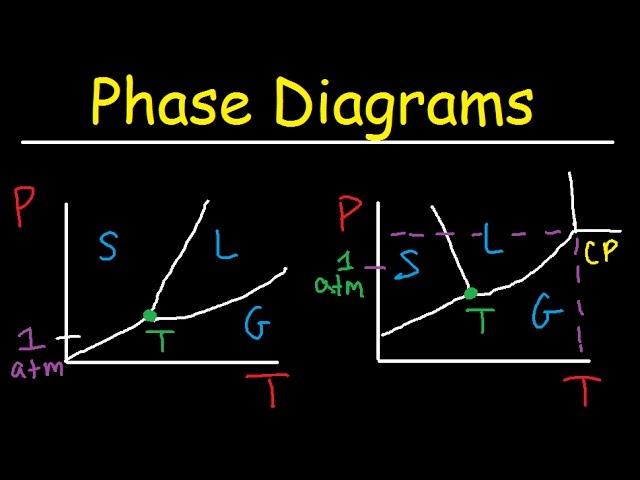
If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Compressibility factor (Z) for a van der Waals real gas at critical point is
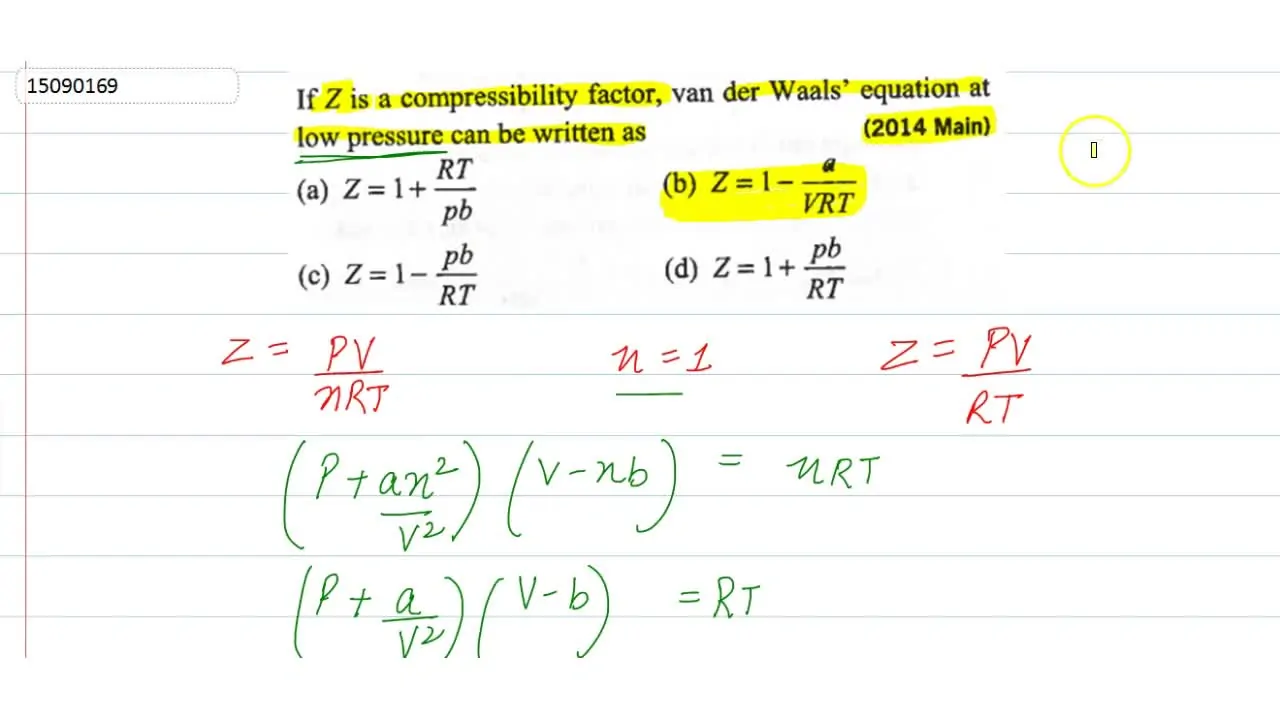
If Z is a compressibility factor, van der Waals' equation at low press

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

If Z is a compressibility factor, vander Waals equation low pressure can be written as [JEEN (0)2=1 Rang (1) Z= 1 + RT Pb (2) Z 2)2=1= = 1 - 2= (3) Z = 1 - 42=1 (4)Z = 1 + VRT

The compressibility factor for definite amount of van der Waals' gas at `0^(@)C` and
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
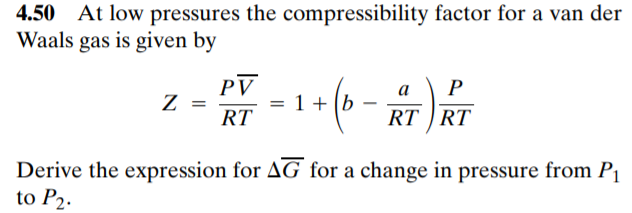
Solved 4.50 At low pressures the compressibility factor for