Ideal Gas Assumptions - Kinetic Theory

When considering a gas as an ideal gas and applying the ideal gas law pV=nRT, we need to make 4 assumptions. (1) The volume of a molecule within the gas is n
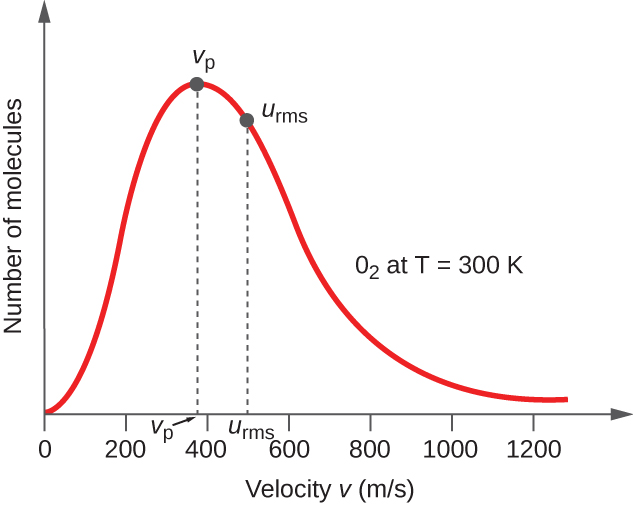
5.4 The Kinetic-Molecular Theory – Chemistry

i.ytimg.com/vi/otZvnZRQ65Y/sddefault.jpg

Boyle's Law - A Level Physics

Revision Notes on Kinetic Theory of Gases

Class 11] Kinetic theory of gases – Physics Handwritten Notes for

2.5 The Ideal Gas (Thermal Physics) (Schroeder)

Describing An Ideal Gas, Moles, Molar Mass, Relative Molar Mass - Kinetic Theory (Lesson 1)

The Kinetic Theory of Gases Temperature as a measure of average

What are the basic assumptions of kinetic theory of gases On their

The kinetic theory of gases: Fundamental assumptions

01 Kinetic Theory of Gases Theory1