117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum

Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum

Solved We begin by showing that the compressibility factor

Chemosensors, Free Full-Text

The compressibility factor a real gas high pressure is:-1 - frac{Pb} {RT}1 + frac {RT} {Pb}11 + frac {Pb} {RT}
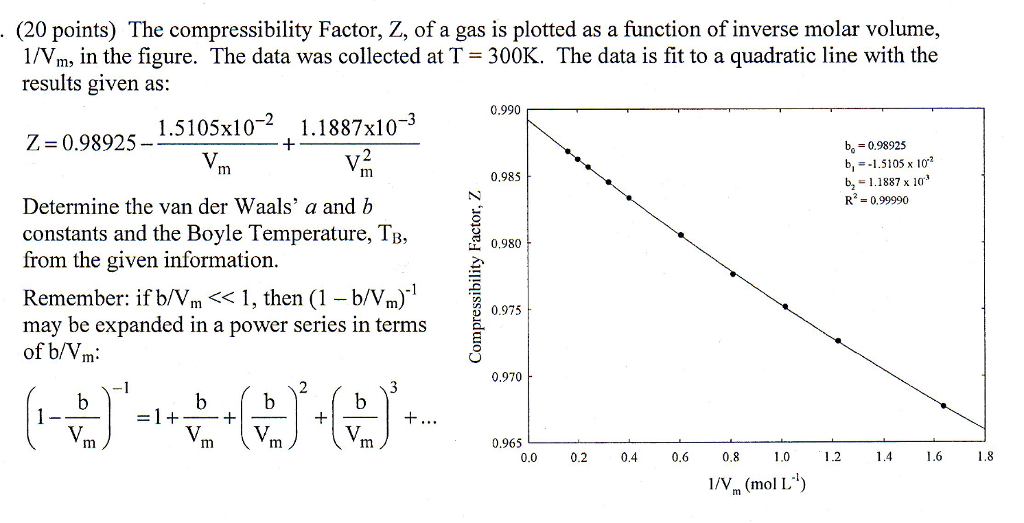
Solved The compressibility Factor, Z, of a gas is plotted as

PCAST Vol 2, Ch 3 Engineering - The FIRE Place

Energies, Free Full-Text

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

The compressibility factor a real gas is BP expressed by, Z=1+ er. The value of B 500 K and 600 bar is 0.0169 L/mol. Therefore the molar volume of the gas 500

Real Gases Introductory Chemistry

Compressibility factor for H(2) behaving as real gas is

PDF) Irreversible myocardial cell injury and early postoperative function of the heart after coronary artery bypass grafting (CABG)
IPCB Publications Database