FDA Enhances Global Patient and Regulatory Collaborations in Oncology

In recognition of World Cancer Day 2024, the FDA and European Medicines Agency will collaborate to spotlight innovative cancer treatment advances for patients.
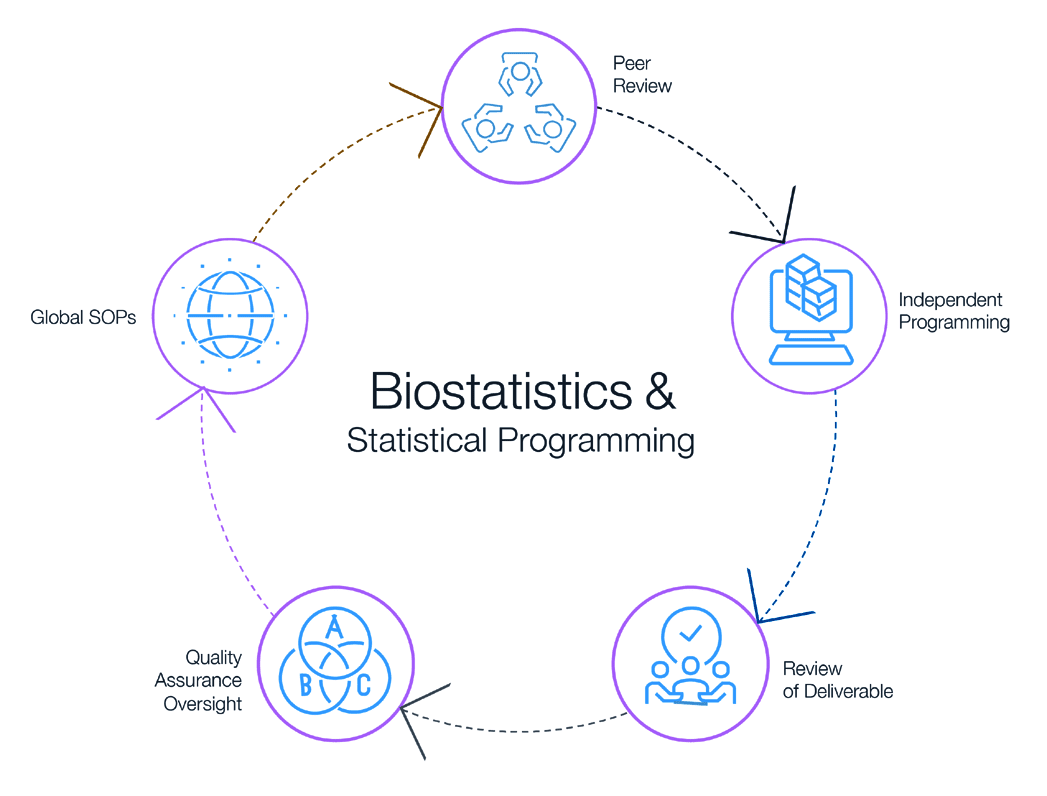
Biostatistics Consulting

Joseph Gibbons (@perevisage) / X

FDA proposes new regulations to increase oversight of Laboratory Developed Tests

FDA releases guidelines to minimise cancer-causing chemicals in drugs - Pharmaceutical Technology

Kirsten Boyd Goldberg posted on LinkedIn

Maria Golovina, MS MBA on LinkedIn: #proud #innovation #leadership

Jan Geissler on LinkedIn: #rarediseaseday #rarecancer #kudos #grateful

FDA's Global Gene Therapy Pilot Program: Will 2024 mark the maturation of gene therapy approvals?

FDA approves pediatric neuroblastoma drug based on Penn State College of Medicine professor's work - Penn State Health News

Project Community
New VQIP Resource - US FDA