The Cottrell Experiment and Diffusion Limitation 3/3

In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

An insight into polyscopoletin electrosynthesis by a quality-by-design approach

The interpretation of small molecule diffusion coefficients: Quantitative use of diffusion-ordered NMR spectroscopy - ScienceDirect
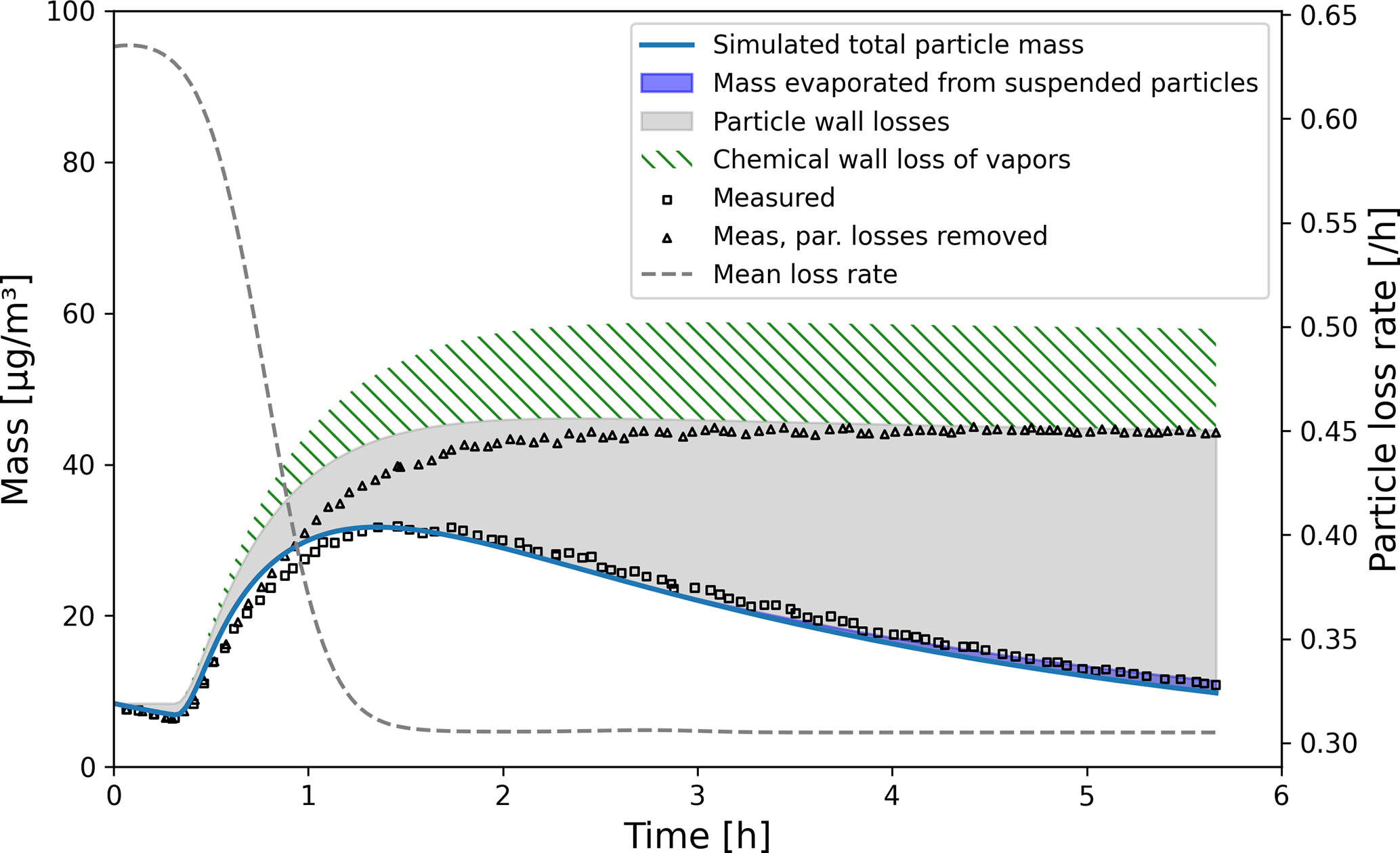
GMD - Atmospherically Relevant Chemistry and Aerosol box model – ARCA box (version 1.2)

More Accurate Measurement of Return Peak Current in Cyclic Voltammetry Using Diffusional Baseline Fitting

Polymers, Free Full-Text
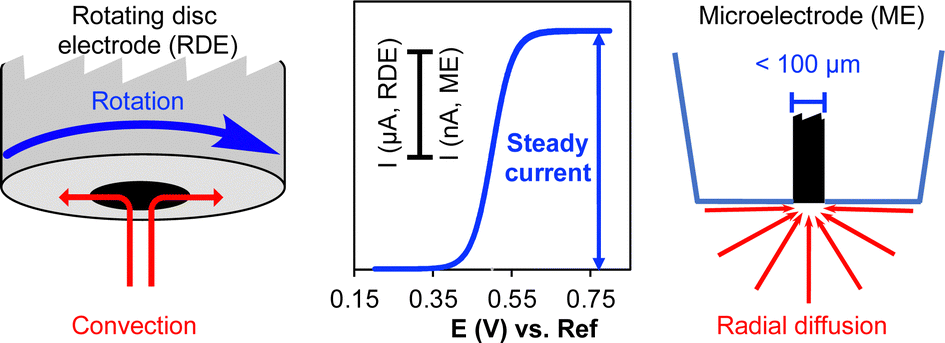
Cyclic voltammetry and chronoamperometry: mechanistic tools for organic electrosynthesis - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00706A
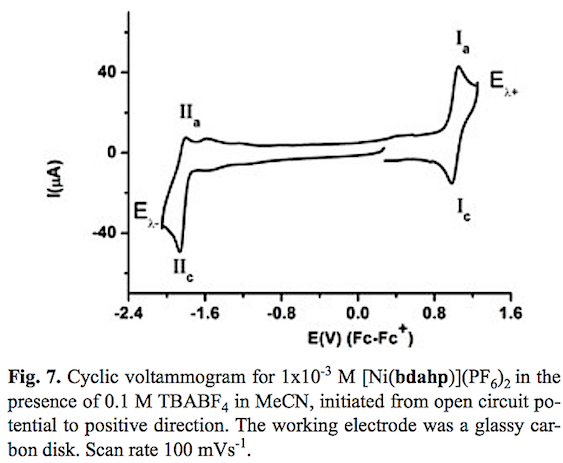
Electrochemical Behavior of Ni(II) Complexes with N2S2 and N6 Ligands as Potential Catalysts in Hydrogen Evolution Reaction

Phase Transformation Lecture 3

Nyquist plot of impedance spectra taken on TLC at three different
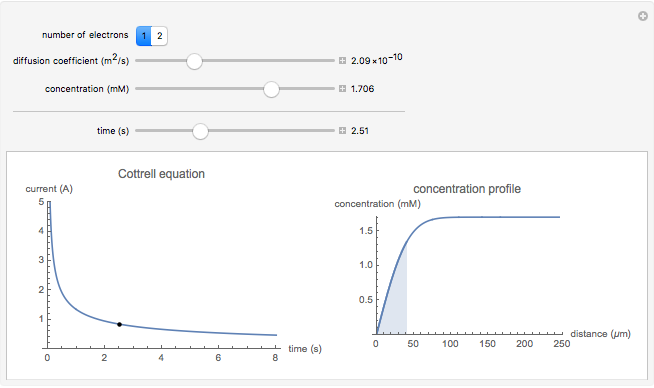
Cottrell Equation for the Potential-Step Experiment - Wolfram Demonstrations Project
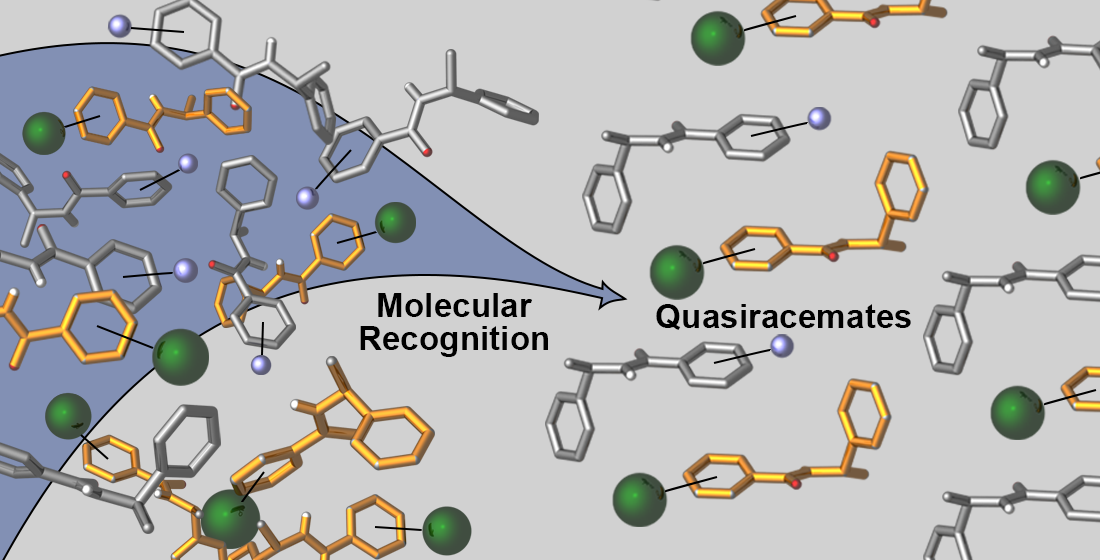
Crystals, Free Full-Text
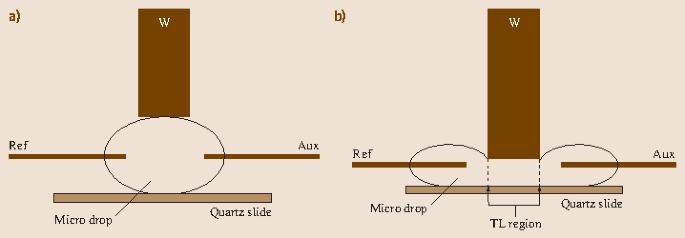
Spectroscopy of Electrochemical Systems

JET – World's Largest Tokamak and its d-t Fusion Experiments Plus TFTR's

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes