Sacituzumab Earns Regular FDA Approval for TNBC - NCI

Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.

Sacituzumab Govitecan Moves to Second-Line Therapy for Metastatic

Therapeutic efficacy of IMMU-132 with different DARs. NCI-N87

PDF) Metastatic Triple-Negative Breast Cancer

Mission Mountain Wilderness
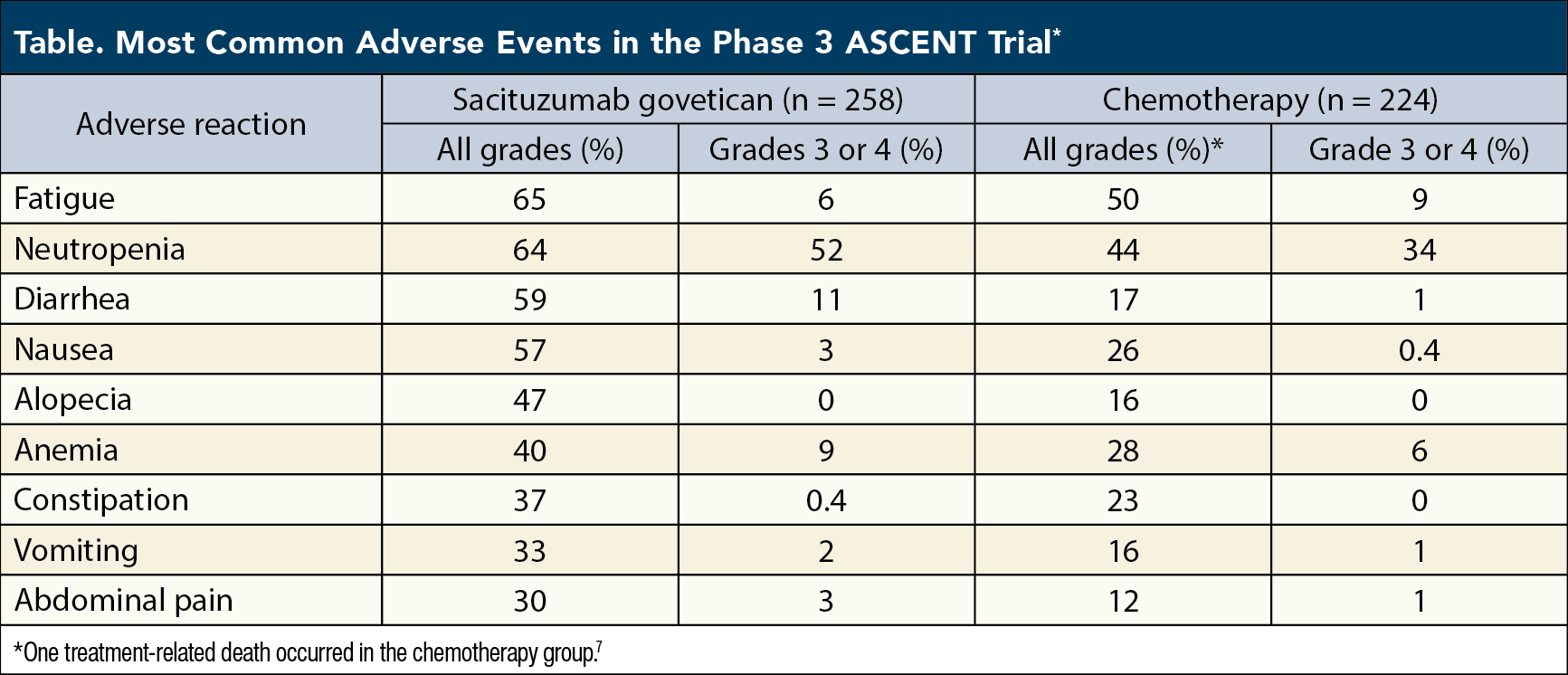
Sacituzumab Govitecan Moves to Second-Line Therapy for Metastatic

Targeted therapy for breast cancer: An overview of drug classes

Triple negative breast cancer and non-small cell lung cancer

Mission Mountain Wilderness
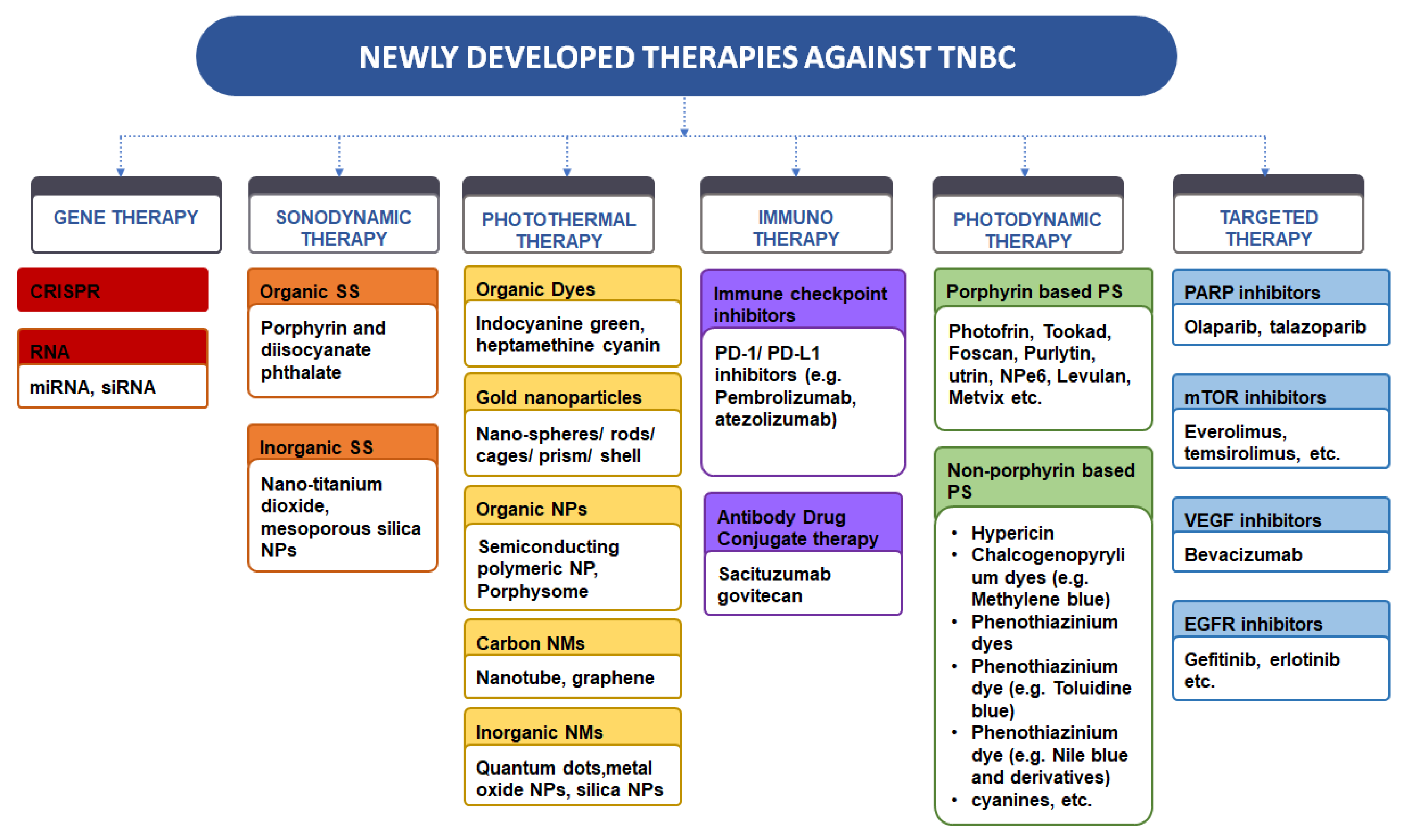
Pharmaceutics, Free Full-Text
Triple Negative Breast Cancer Archives - Tigerlily Foundation

Biomolecules, Free Full-Text

Sacituzumab govitecan in metastatic triple negative breast cancer

Triple negative breast cancer: Pitfalls and progress