Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations

This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.

Multi-Society Consensus Conference and Guideline on the Treatment of Gastroesophageal Reflux Disease (GERD) - A SAGES Publication
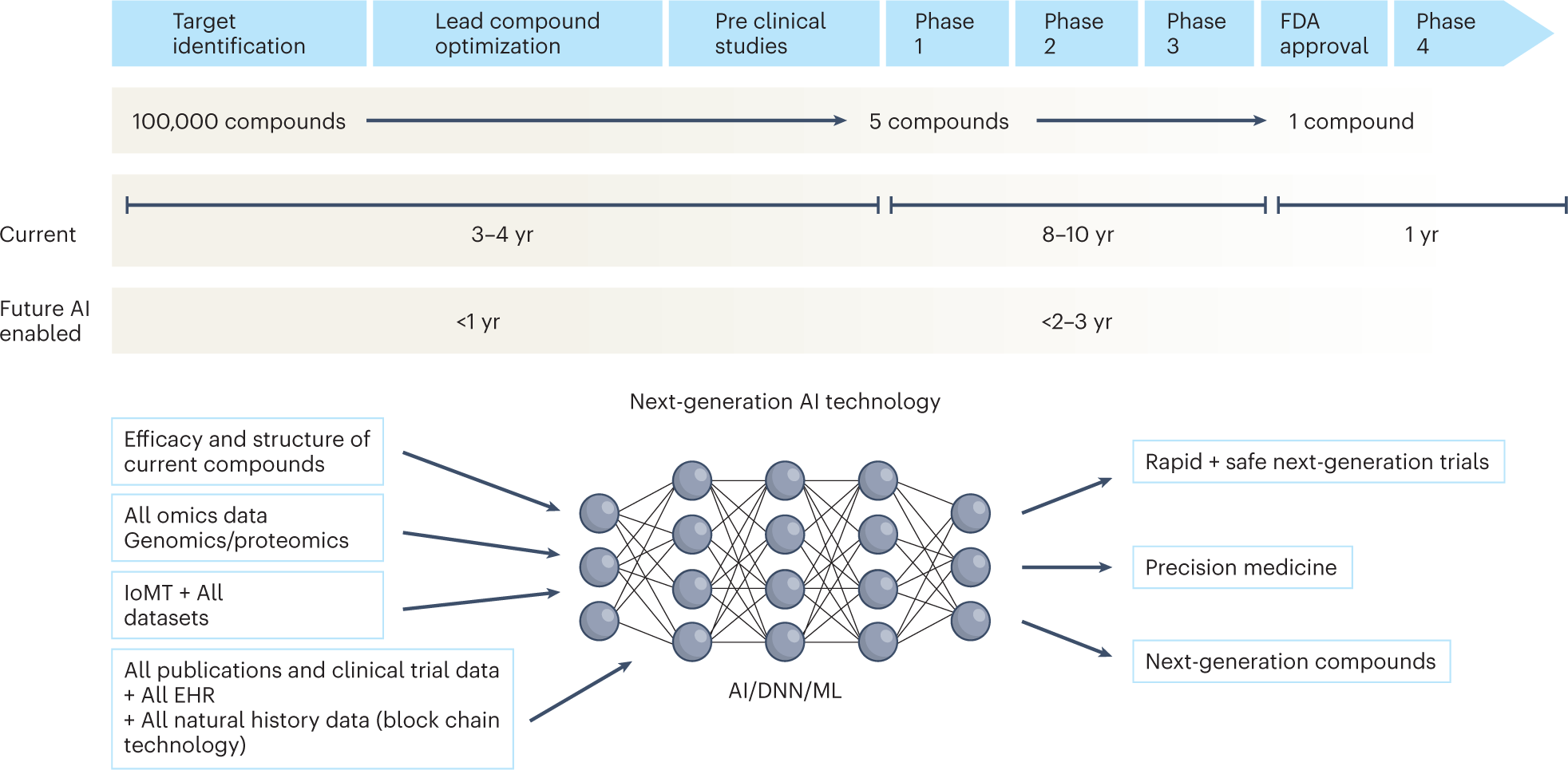
The next generation of evidence-based medicine

Current Medical Device Regulations in Canada
Decentralized Procedure for Marketing Authorization in EU (1-2) 5.

Demystifying The Investigational Device Exemption Process - Healthcare - United States

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Ultimate Guide to UDI for Medical Devices
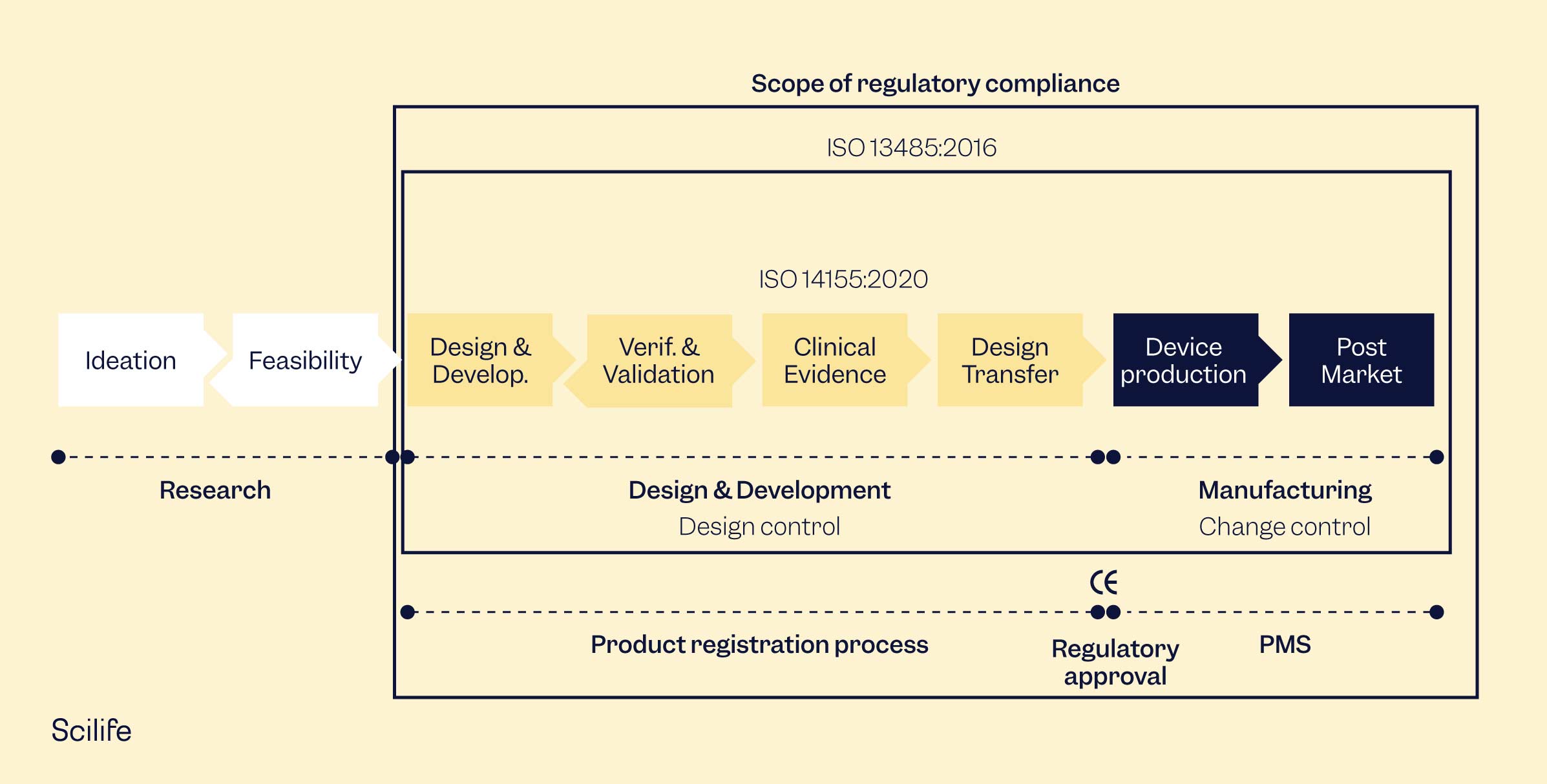
A Guide to Bringing a Medical Device to Market

Medical device submissions: Placing a medical device on the market
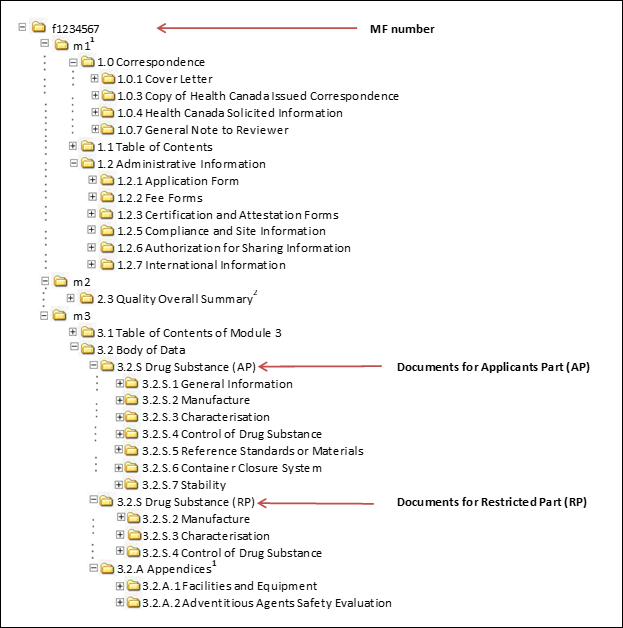
Guidance document: preparation of regulatory activities in non-eCTD format
.png)
eCRF: Electronic Case Report Form in Clinical Trials - Essential Guide

Medical device - Wikipedia

Essential Documents Required for Conducting Clinical Trials

RQM+ Medical Device and In Vitro Diagnostic Blog