Derive an expression for the compression factor of a gas tha
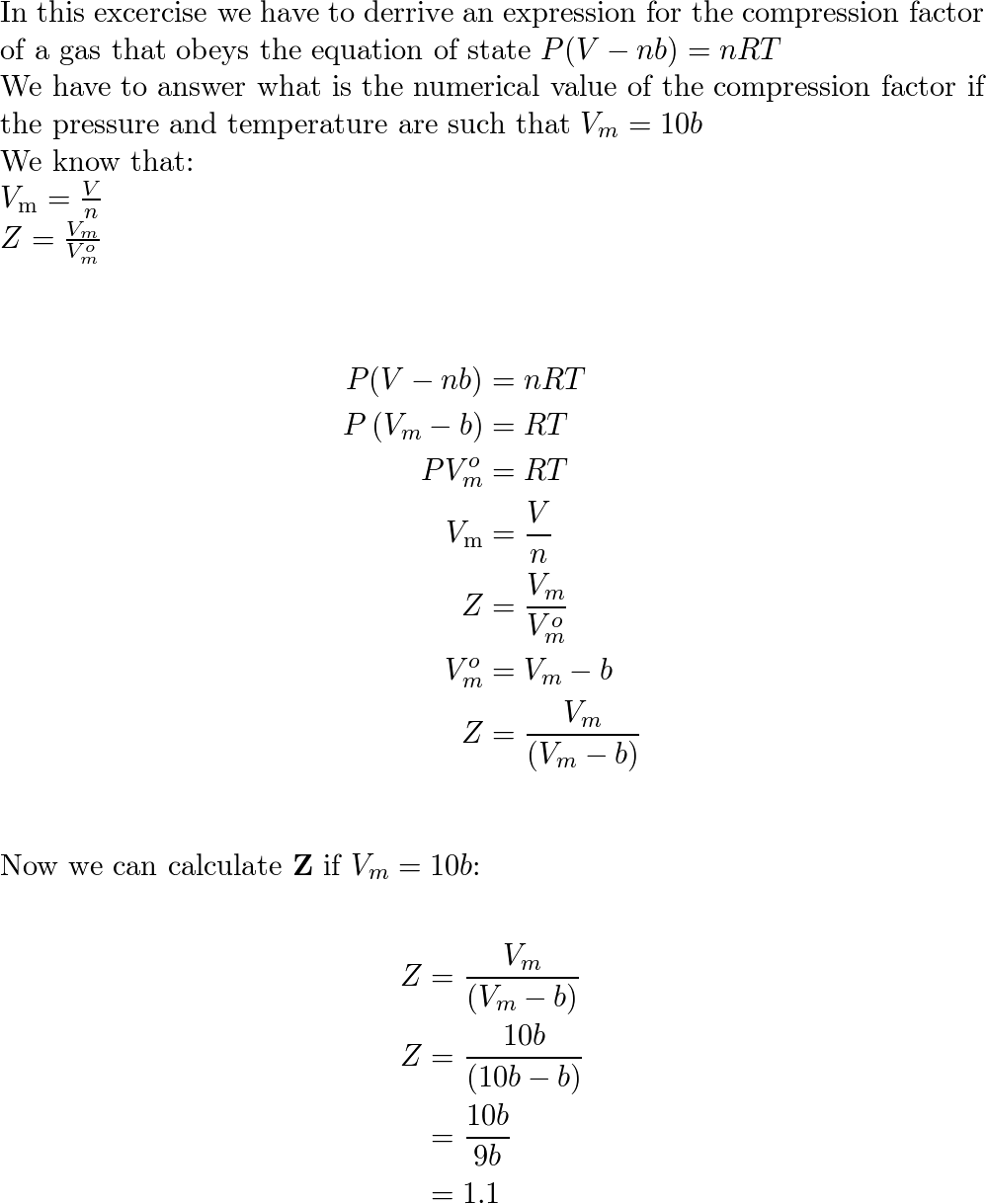

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Atkins - Cap1 - Ejerciciosatkins - Cap1peter Atkins - Atkins Physical Chemistry (2006, Oxford University Press) PDF, PDF, Gases

Student Solutions Manual to Accompany Atkins' Physical Chemistry [11 ed.] 9780198807773, 0198807775
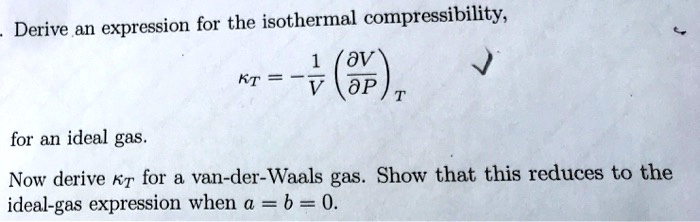
SOLVED: Derive an expression for the isothermal compressibility, KT = (0), for an ideal gas. Now derive Rr for van der Waals gas. Show that this reduces to the ideal gas expression

Assignment 2 - Physical Chemistry, CHEM 3615, Assignments Physical Chemistry
Solved] Sketch how the compression factor z of an ideal and real gases

CH-Physical Chemistry(8th ed)[英语]Atkins
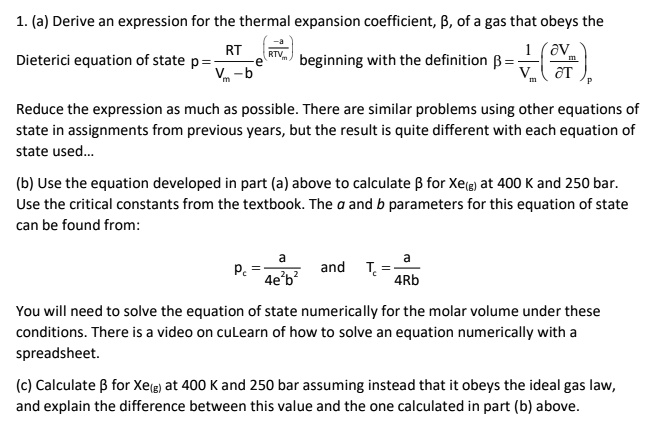
SOLVED: (a) Derive an expression for the thermal expansion coefficient of a gas that obeys the Dieterici equation of state, beginning with the definition α = (1/V)(∂V/∂T). Reduce the expression as much

Atkins' Physical Chemistry [11th ed.] 0198769865, 9780198769866
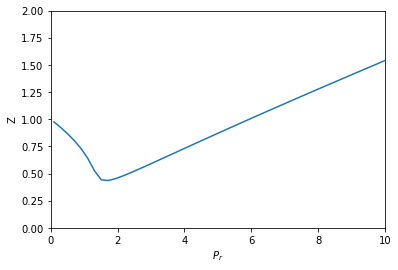
Compressibility factor variation from the van der Waals equation by three different approaches

SOLVED: Devise an expression for the compression factor of a gas that obeys the equation PVm - b = RT, where b and R are constants. If the pressure and temperature are
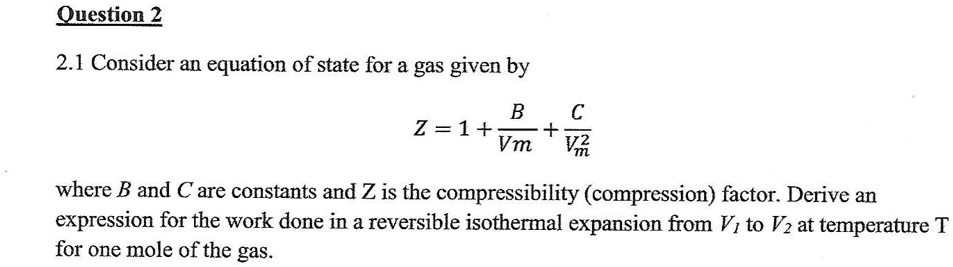
Solved Deduce The Correct Expression For Calculating VR, 52% OFF

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics