Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
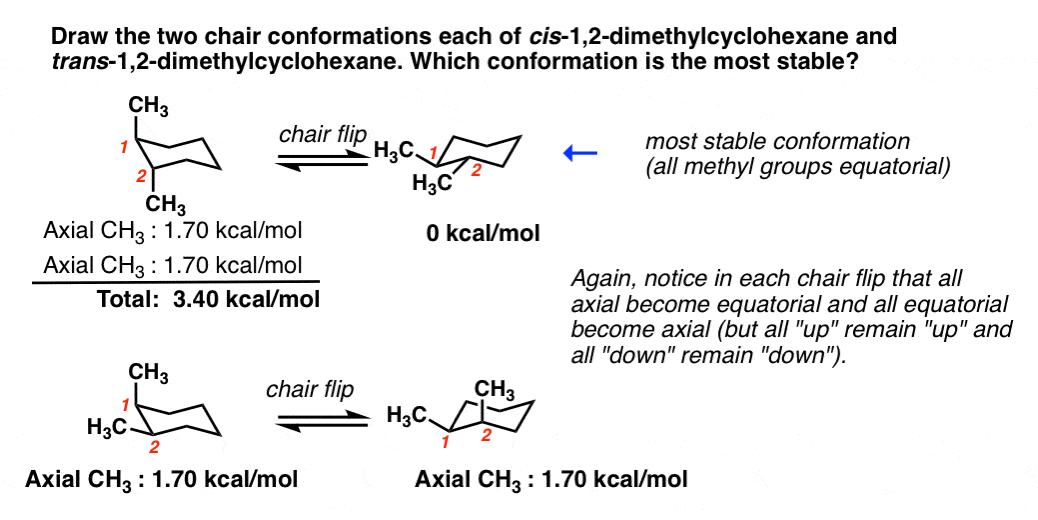
To determine chair conformation stability, add up the "A-Values" for each axial substituent. The lower that number is, the more stable the chair.

Ring Inversion - an overview
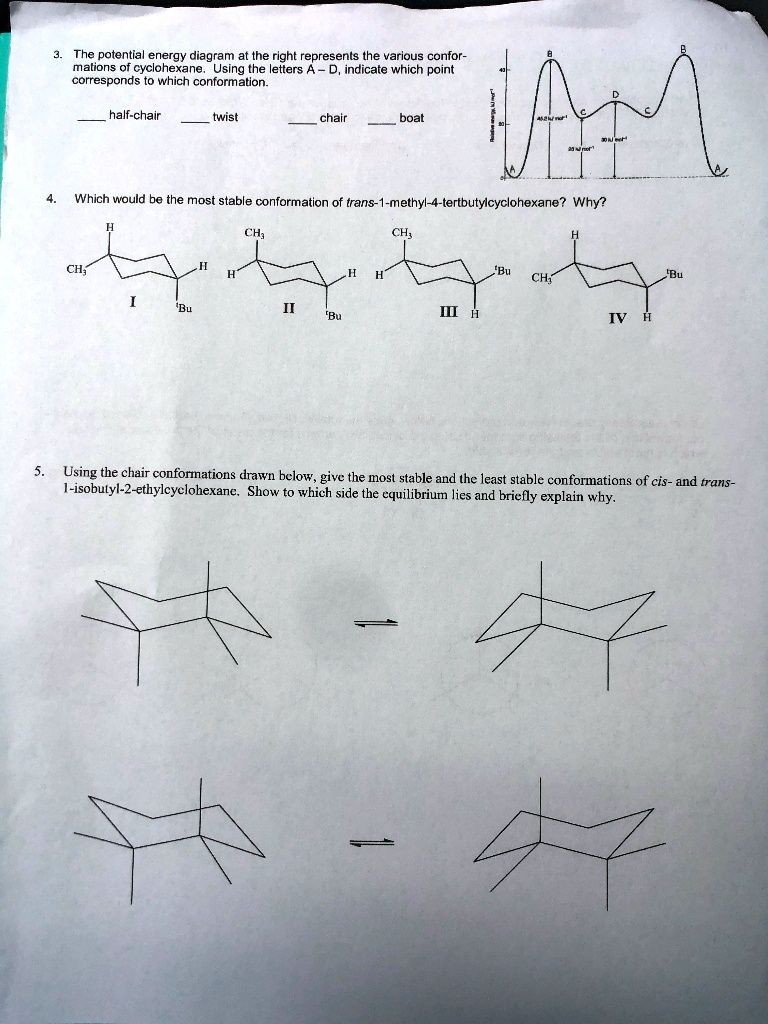
SOLVED: The potential energy diagram at the right represents the various conformations of cyclohexane. Using the letters A, B, C, and D, indicate which point corresponds to which conformation. A - half-chair
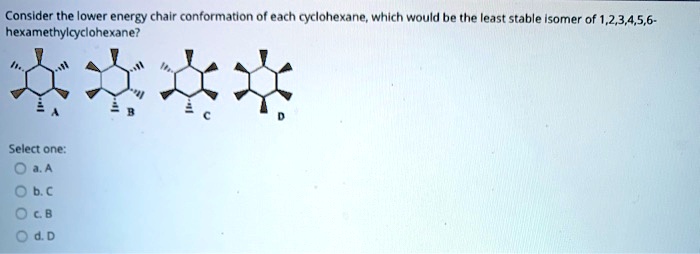
SOLVED: Consider the lower energy chair conformation of each cyclohexane; which would be the least stable Isomer of 1,2,3,4,5,6: hexamethylcyclohexane? Select one
OrgoSolver
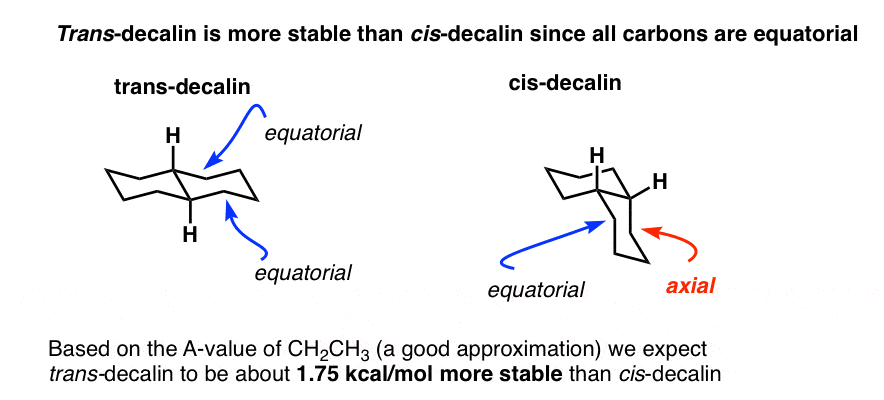
Fused Rings: Cis and Trans Decalin – Master Organic Chemistry
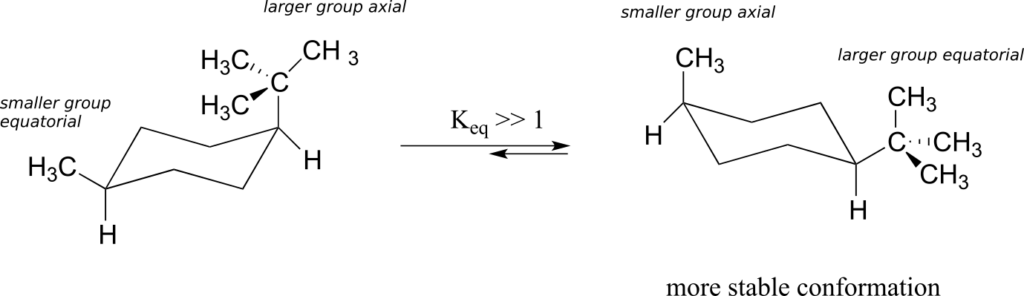
8.2 Conformation of Organic Compounds – Chemical Bonding and Organic Chemistry

Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
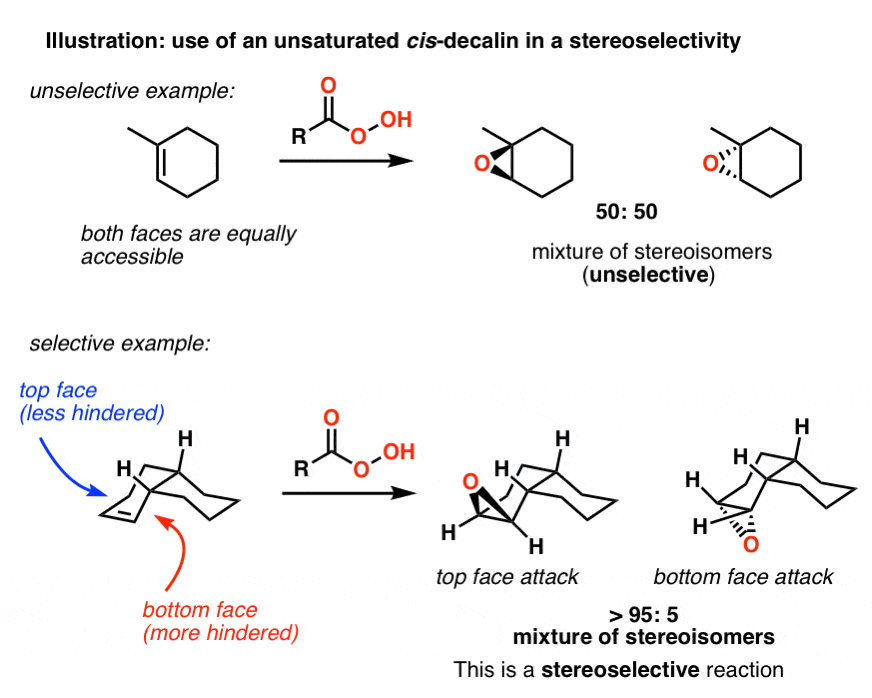
Fused Rings: Cis and Trans Decalin – Master Organic Chemistry
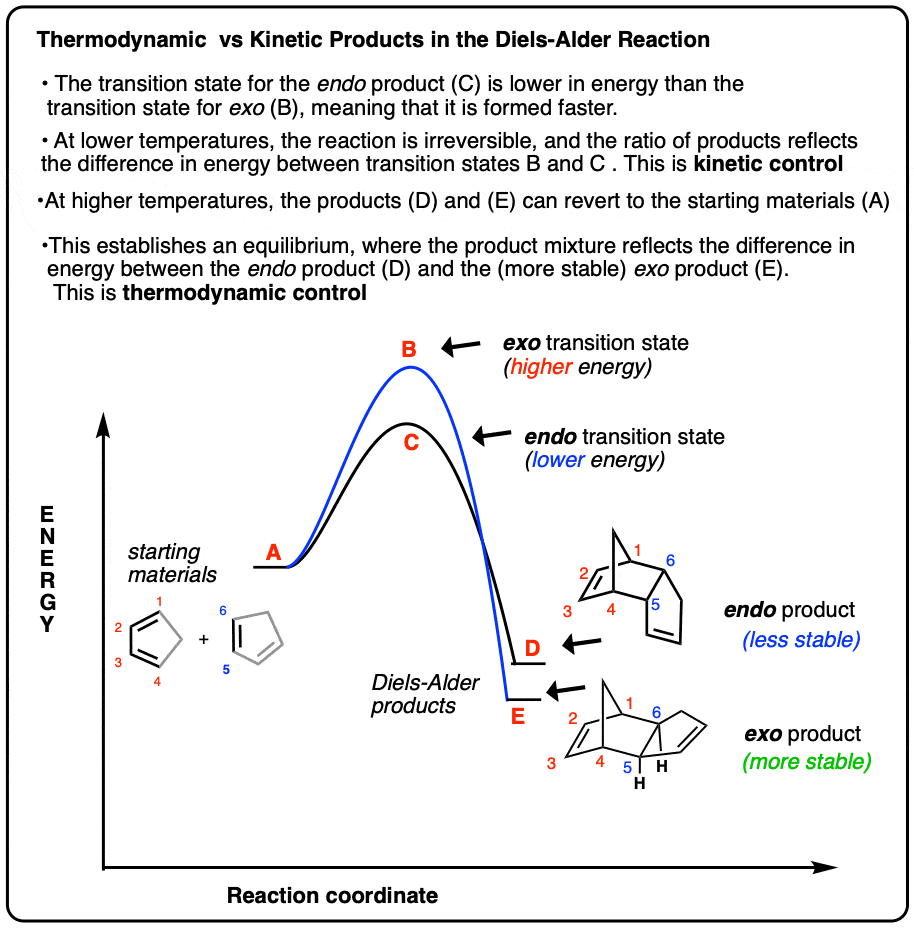
Kinetic and Thermodynamic Control in the Diels-Alder Reaction
What are the stable conformations of cis and trans 1,2 cyclohexane diol and which one will readily form ketal with acetone? - Quora
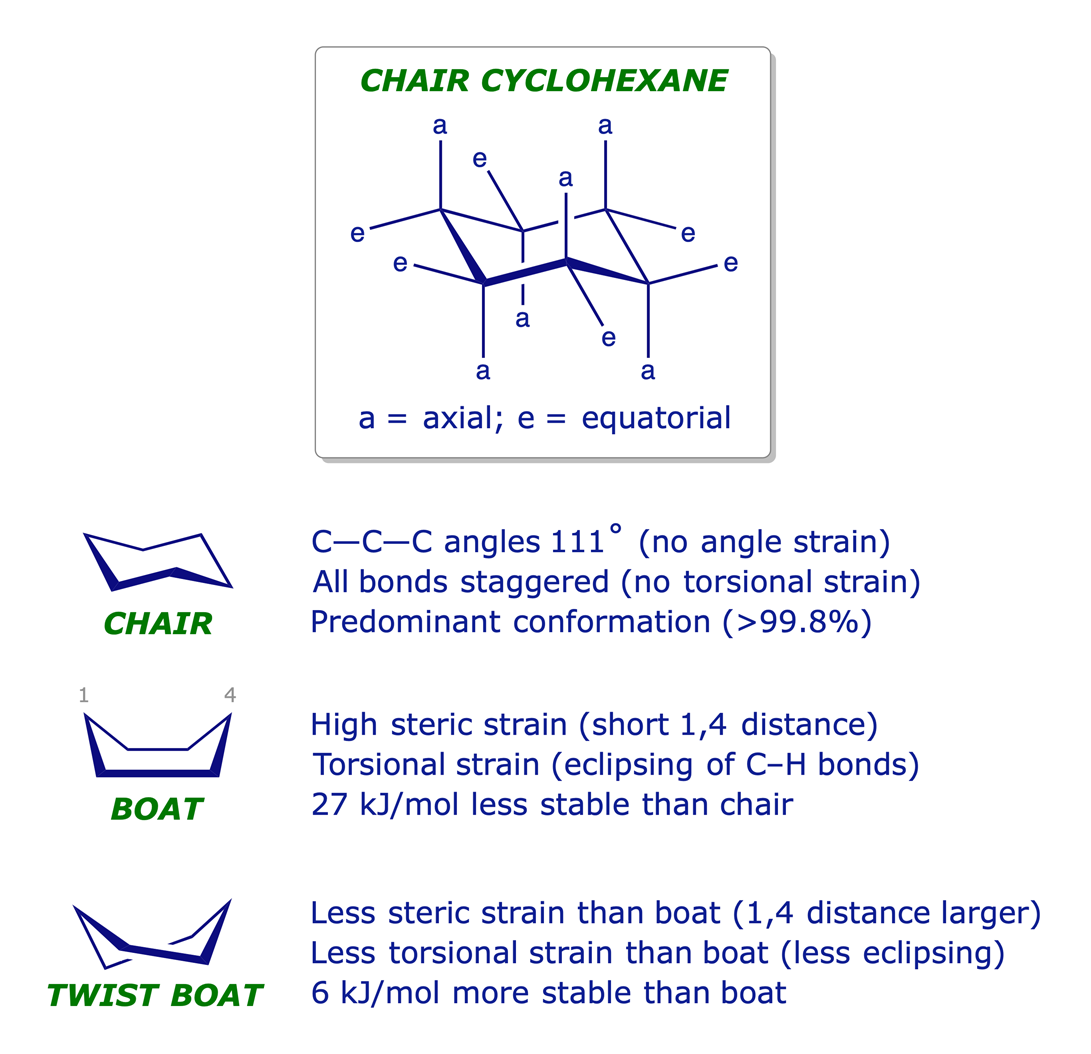
Cyclohexane
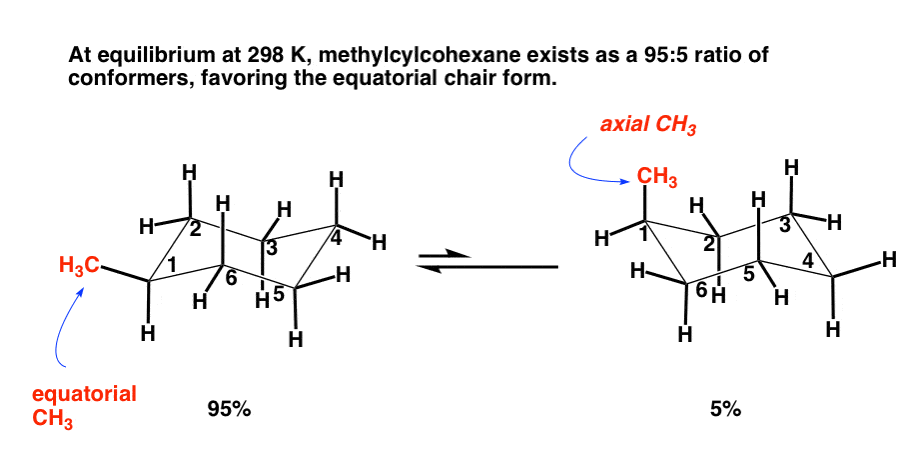
Substituted Cyclohexanes: Axial vs Equatorial – Master Organic Chemistry
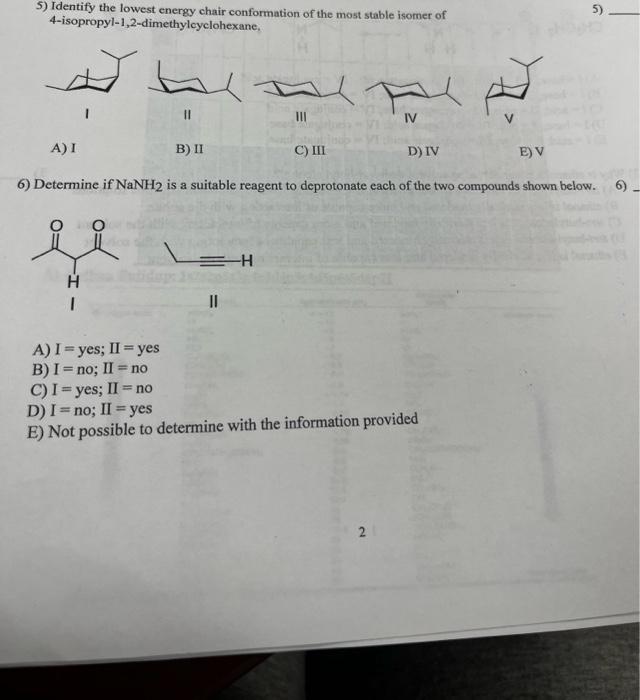
Solved 5) Identify the lowest energy chair conformation of
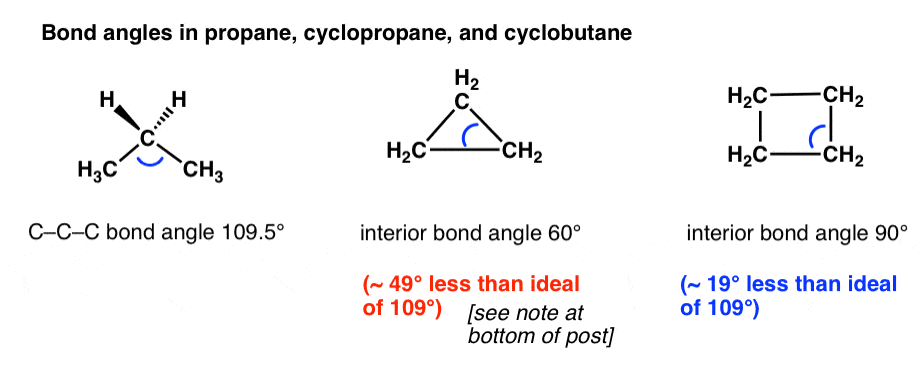
Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
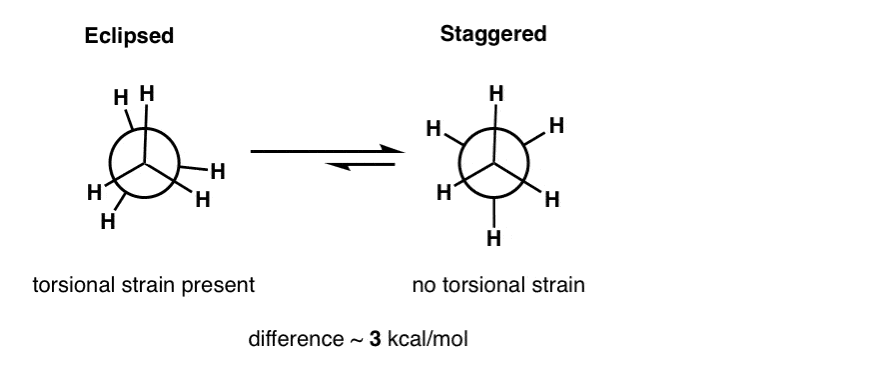
Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane