Cordis Closure by Cordis

A Portfolio of Vascular Closure Devices
Expired 2020 The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.

New CORDIS EX500 ExoSeal Vascular Closure Device 5F (#E1) Expired EX500 ExoSeal Vascular Closure Device 5F (#E1) Expired For Sale - DOTmed Listing

CORDIS: 504-608X

Cordis Closure by Cordis
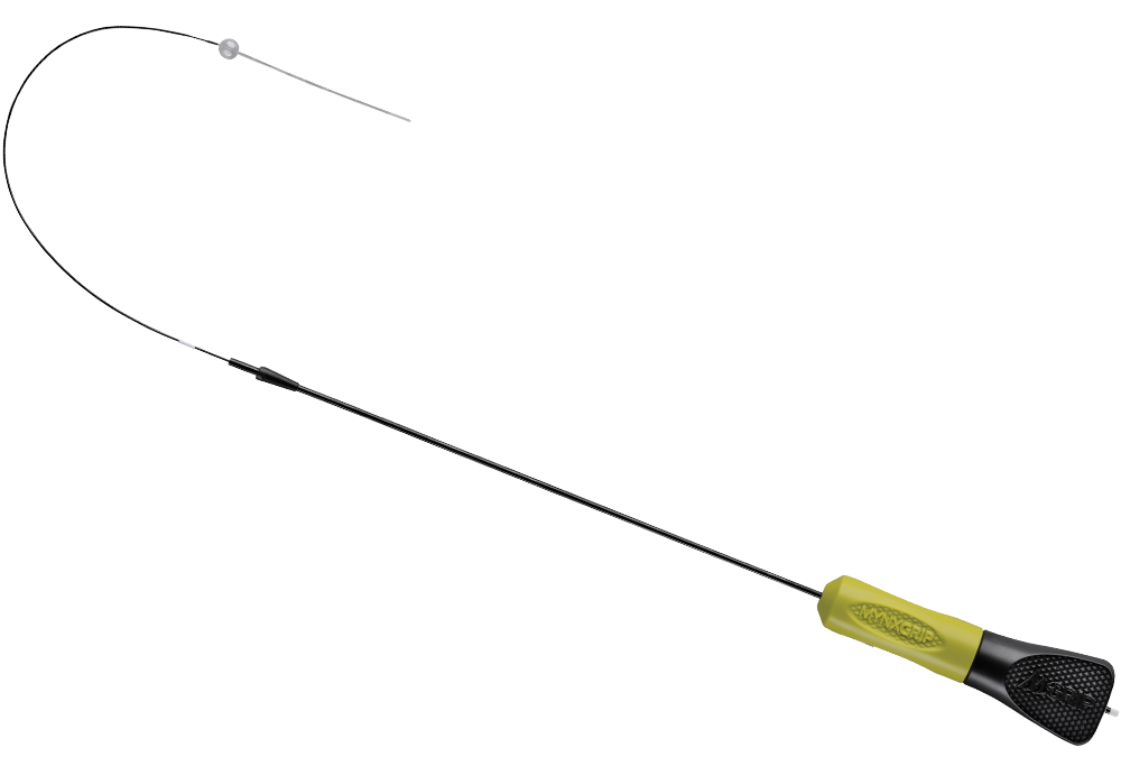
Cordis Closure by Cordis

CORDIS EX700 ExoSeal Vascular Closure Device, 7F (X)

Cordis on LinkedIn: #cordis #connectwithcordis
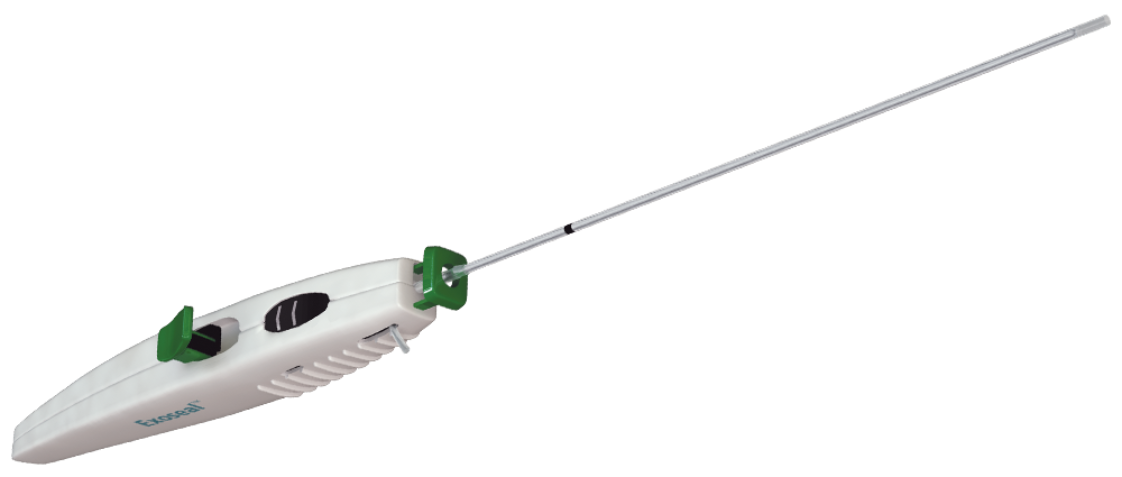
Cordis Closure by Cordis
MYNX Control Vascular Closure Device, 6 Fr/7 Fr Size

CORDIS: MX6760

FDA Clears New Cordis Vascular Closure Device
__14076.1683665559.jpg)
ExoSeal Cordis Device for TRAINING ONLY EX600CE

Cordis, a Cardinal Health company

Exoseal Femoral Access Vascular Closure Device

New CORDIS Box of 5 Cordis ExoSeal 6F EX600 Exp.08-2021 (#01) EX600 ExoSeal 6F Disposables - General For Sale - DOTmed Listing #3749728
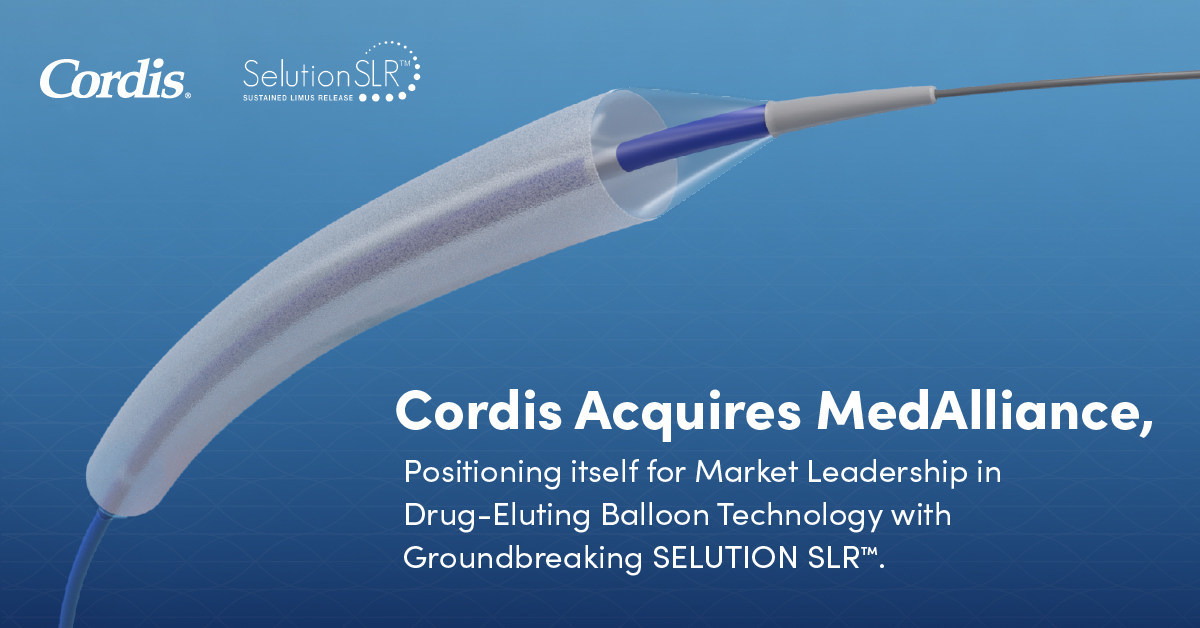
Cordis Announces Acquisition of MedAlliance, Positioning Itself for Market Leadership in Drug-Eluting Balloon Technology

Serial photographs of Case 1 (a) Newborn period ectopia cordis. (b)